Cobalt(II) Chloride Catalyzed One Pot Synthesis of 2-Substituted and 3-Substituted-4(3H)-Quinazolinones
Aayesha Nasreen*, Rita M. Borik
Department of Chemistry, College of Sciences, Jazan University, 6811-Arroudah P.O Box No. 2097, Jazan 82724-3750, Saudi Arabia
DOI : http://dx.doi.org/10.13005/ojc/300249
Article Received on :
Article Accepted on :
Article Published : 12 Apr 2014
Cobalt(II) chloride (10mol%) was found to be an efficient catalyst for one pot synthesis of variety of 2-substituted-4(3H)quinazolinones by condensation of anthranilamide and aldehydes and synthesis of 3-substituted-4(3H)quinazolinones by condensation of anthranilic acid, orthoester, primary amines at reflux giving good to excellent yields (75-95%).
KEYWORDS:cobalt(II) chloride; 2-substituted and 3-substituted 4(3H)quinazolinones; anthranilamide; aldehydes, anthranilic acid; HC(OEt)3; anilines.
Download this article as:| Copy the following to cite this article: Nasreen A, Borik M. R. Cobalt(II) Chloride Catalyzed One Pot Synthesis of 2-Substituted and 3-Substituted-4(3H)-Quinazolinones. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Nasreen A, Borik M. R. Cobalt(II) Chloride Catalyzed One Pot Synthesis of 2-Substituted and 3-Substituted-4(3H)-Quinazolinones. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=2910 |
INTRODUCTION
The quinazolinone core and its derivatives form an important class of compounds, as they are present in a large family of products with broad biological activities. 4(3H)-quinazolinones are versatile nitrogen heterocyclic compounds, displaying a broad spectrum of biological and pharmacological activities such as anti-fungal,1 anti-tumour,2 hypotensive,3anti-cancer,4,5anti-HIV,6anti-inflammatory,7anti-bacterial,8 etc. Furthermore, 4(3H)-quinazolinones substituted at 2,3-position derivatives play a pivotal role in the hypertensive activity.9,10 Several bioactive natural products such as febrifugine and isofebrifugine contain quinazolinone moieties with potential anti-malarial activity11 Similarly quinazolinone containing moieties have been known as tyrosine kinase inhibitors,12 dihydrofolate reductase inhibitors,13 and tubulin polymerization inhibitors.14 Due to their wide range of applications these compounds have received a great deal of attention in connection with their synthesis. Many reagents have been reported in the literature,15-26 for the synthesis of 4(3H)-quinazolinone derivatives. Among these methods 2-substituted-4(3H)quinazolinones have been synthesized from anthranilamide and aldehydes using NaHSO3,16a DDQ,16b CuCl2,20 FeCl3.6H2O,25a Cu(NO3)2.3H2O,25b and 3-substituted-4(3H)quinazolinones were recently prepared by using various catalysts such as Yb(OTf)3,15hSilicagel/FeCl3,17a Nafion-H,17b (a perfluorinated resin supported sulfonic acid), under microwave irradiation, Zn(ClO4)2,18 heteropolyacids such as H3PW12O40.13H2O,19 La(NO3)3.6H2O,21 Bi(TFA)3-FeCl4.22 In continuation of our work to develop new organic transformations,27-29 we report here in that cobalt(II) chloride which acts as a mild lewis acid might be useful and inexpensive catalyst for the synthesis of 2-substituted-4(3H)quinazolinones and 3-substituted-4(3H)quinazolinones. Although cobalt(II) chloride has been extensively used as a mild catalyst for a variety of organic transformations,30-32 there are no examples of the use of cobalt chloride as catalyst for the synthesis of 2-substituted-4(3H)-quinazolinones and 3-substituted-4(3H)quinazolinones. In the present study 2-substituted-4(3H)quinazolinones and 3-substituted-4(3H) quinazolinones are synthesized and the results are presented here.A high yield condensation of anthranilamide, aldehyde (alkyl, aryl, heteroaryl) acetonitrile at reflux in the presence of catalytic amount of co(II) chloride, 2-substituted-4(3H)quinazolinones are obtained(scheme-1). 3-substituted-4(3H)quinazolinones are obtained from the reaction of anthranilic acid, triethyl ortho-formate (HC(OEt)3) and anilines at reflux in the presence of catalytic amount of co(II) chloride, and acetonitrile as solvent(scheme-2).
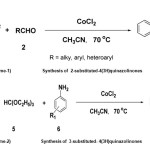 |
Scheme 1 and 2 Click here to View Scheme |
Experimental
Material and Methods
Chemicals were purchased from Merck and Fluka and directly used for the synthesis. Thin layer chromatography (TLC): precoated silica gel plates (60 F254, 0.2mm layer; E. Merck). 1H NMR (Avance 300 MHz) spectra were recorded in DMSO using TMS as internal standard. Chemical shifts (δ) are reported in ppm, Melting points (M.P.) were determined on a Fischer-Johns melting point apparatus. IR and MS were recorded on a Thermo Nicolet Nexus 670 FT-IR Spectrometer and Finnegan MAT 1020 Mass spectrometer operating at 70 ev. Elemental analyses were performed on a Perkin Elmer 2400series II Elemental CHN analyzer.
General procedure for the synthesis of 2-substituted-4(3H)quinazolinones
To a mixture of anthranilamide (1mmol) in acetonitrile (5mL), CoCl2 (10mol%) was added to the appropriate aldehyde (1.3mmol) was refluxed at 70oC for the time specified in (Table-3) for each substrate, after completion of the reaction as indicated by TLC the reaction mixture was allowed to cool and quenched with NaHCO3 followed by brine solution and extracted with ethyl acetate dried over Na2SO4, concentrated under vacuum, and the crude mixture was purified by column chromatography (hexane:ethylacetate 7:3) to afford the corresponding pure 2-substituted-4(3H)quinazolinones 3a-3m (Table-3). All the products are well characterized by spectral analysis (IR,1HNMR, MS) and were found to be identical those reported in the literature.20, 25
General procedure for the synthesis of 3-substituted-4(3H)quinazolinones
To a mixture of anthranilic acid (1mmol) in acetonitrile (5mL) triethyl orthoformate (HC(OEt)3) 1.5mmol), the appropriate aniline (1.3mmol) and CoCl2 (10mol%) was refluxed at 70 oC for the time specified in (Table-4) for each substrate, after completion of the reaction as indicated by TLC the reaction mixture was allowed to cool and quenched with NaHCO3 followed by brine solution and extracted with ethyl acetate dried over Na2SO4, concentrated under vacuum, and the crude mixture was purified by column chromatography (hexane:ethylacetate 7:3) to afford the corresponding pure 3-subtituted-4(3H)quinazolinones 7a-7o (Table-4). All the products are well characterized by spectral analysis (IR,1HNMR, MS) and were found to be identical with those reported in the literature.14b, 17-19, 21, 26
Spectral data for selected compounds: 2-Phenyl-4(3H)-quinazolinone (3a)
mp (oC)25 237-239, IR(KBr): 679, 1458, 180, 1356, 1037, 965,738 cm-1; 1HNMR(300MHz, DMSO-d6) 7.56-7.62 (m, 4H),7.75-7.84 (m, 2H), 8.18-8.53 (m, 3H),12.46 (s,1H, NH) ppm; Anal. Calcd. for C14H10N2O: C, 75.68; H, 4.51; N, 12.68; O, 7.5. Found: C, 75.67; H, 4.48; N, 12.50. MS(EI): m/z (%) 222(M+).
(4-Hydroxyphenyl-3-methoxy)-4(3H)-quinazolinone (3c)
mp(oC)25267-268, IR(KBr) 1664, 1578, 1545, 1483, 1280, 1248, 1027, 1025, 866, 770 cm-1; 1HNMR (300MHz, DMSO-d6) 3.94 (s, 3H, CH3), 6.98 (d, 1H, J=8:3Hz, Ph), 7.44 (t, 1H,J=7:4Hz, Ph), 7.75 (d, 1H, J=8:0Hz, Ph), 7.77 (d, 1H, J=8:3Hz,Ph), 7.80 (s, 1H, Ph), 7.86 (t, 1H, J=7:2Hz, Ph), 8.17 (d, 1H, J=7:8Hz, Ph), 9.85 (s, 1H, OH); 12.38(s, 1H, NH) ppm; Anal. Calcd for C15H12N2O3: C, 67.20; H, 4.53; N, 10.49. Found: C, 67.29; H, 4.60; N,10.30; MS (EI): m/z(%) 268.(M+)
(4-Nitrophenyl)-4(3H)-quinazolinone (3i)
mp(oC)25 >300, IR(KBr): 1682, 1609, 1590, 1523, 1469, 1348, 1151, 949 , 865, 772, 707, 556 cm-1; 1HNMR (300MHz, DMSO-d6) 7.59 (t, 1H, J=7:5Hz, Ph), 7.80 (d, 1H, J=8:0Hz, Ph), 7.89 (t, 1H,J=7:6Hz, Ph), 8.19 (d, 1H, J=8:1Hz, Ph), 8.38 (d, 2H, J=9:1Hz, Ph), 8.43 (d, 2H, J=9:1Hz, Ph) 12.16(s, 1H, NH) ppm; Anal. Calcd for C14H9N3O3: C, 62.94; H, 3.40; N,15.72, Found: C, 62.79; H,3.56; N, 15.89; MS (EI): m/z(%) 267(M+).
(Furyl)-4(3H)-quinazolinone (3f)
mp(oC)25 219–221, IR(KBr): 1663, 1618, 1569, 1457, 1330, 969, 775, 757cm-1; 1HNMR(300MHz, DMSO-d6) 6.73(dd, 1H, J=3:4, 1.3Hz), 7.49 (t,1H, J=7:5Hz, Ph), 7.59 (d, 1H, J=3:4Hz), 7.68 (d, 1H, J=8:1Hz, Ph), 7.81 (t, 1H, J=7:6Hz, Ph), 7.97 (d, 1H, J=1:3Hz),8.11 (d, 1H, J=7:8Hz, Ph), 12.68 (s, 1H, NH) ppm; Anal. Calcd for C12H8N2O2: C, 67.98; H,3.76; N, 13.22. Found: C, 67.86; H, 3.79; N, 13.26; MS(EI): m/z (%) 212(M+).
Phenyl-4(3H)-quinazolinone (7a)
mp(oC)19 139, IR (KBr): 1699, 1598, 1037, 1290, 1463,cm-1; 1HNMR(300MHz, DMSO-d6): 8.34 (d, J =7.6 Hz, 1H), 8.16 (s, 1H), 7.72-7.78 (m, 2H), 7.51 (t, J=7.31 Hz, 1H) and 7.28-7.36 (m, 5H) ppm; Anal. Calcd. for C14H10N2O:C,75.69; H,4.52; N, 12.65; O,7.2. Found: C,75.60; H,4.44; N,12.59; MS(EI): m/z (%) 222 (M+).
(4-Bromophenyl)-4(3H)-quinazolinone (7c)
mp(oC)19186, IR(KBr):1692,1605, 1456, 1070cm-1; 1HNMR(300MHz, DMSO-d6): 8.43(d, J = 8.6 Hz, 1H), 8.12 (s, 1H) and 7.95-7.21 (m, 7H) ppm; Anal. Calcd. for C14H9BrN2O: C, 55.83; H, 3.09; Br, 26.55; N, 9.30. Found: C, 55.92; H, 3.07; N, 9.31; MS(EI): m/z(%), 300(M+).
(4-Methylphenyl)-4(3H)-quinazolinone (7f)
mp(oC)19 148, IR(KBr): 1690, 1578, 1603, 1457cm-1; 1HNMR(300MHz,DMSO-d6): 8.29 (d, J = 7.6 Hz, 1H), 8.11 (s, 1H), 7.69-7.71 (m, 2H),7.40(t, J= 7.2 Hz, 2H), 7.16 (d, J= 7.6 Hz, 2H), 7.28 (d, J= 7.6 Hz, 2H) and 2.26 (s, 3H)ppm; Anal. Calcd. for C15H12N2O: C, 76.25; H, 5.17; N, 11.88; O, 6.97. Found: C, 76.29; H, 5.16; N, 11.77; MS(EI): m/z (%) 236 (M+).
(2,5-Dichlorophenyl)-4(3H)-quinazolinone (7h)
mp(oC)19 146-148 IR(KBr):1673, 1604,1578 1450cm-1; 1HNMR(300MHz, DMSO-d6): 8.43(d, J= 7.6 Hz, 1H), 8.13 (s, 1H), 7.69-7.72 (m, 2H),7.50 (t, J = 7.3 Hz, 1H), 7.38 (d, J= 7.6 Hz, 2H), 7.25 (d, J= 8.6 Hz, 2H)ppm; Anal.Calcd.for C14H8Cl2N2O: C,57.77; H, 2.79; Cl, 24.36; N,9.62. Found:C, 57.92; H,3.02; N, 9.35; MS(EI): m/z(%), 291(M+).
(pyridin-2-yl)-4(3H)-quinazolinone (7i)
mp(oC)18 132-133 IR(KBr): 3061,1684, 1608,1474, 1435, 1328, 1290, 1256, 915, 870, 771 cm-1 1HNMR ((300MHz, DMSO-d6); 8.65 (t, J= 7.3Hz, 2H), 8.40 (s,1H),7.90-7.92 (m, 2H), 7.78-7.84 (m, 2H), 7.54-7.57 (m, 1H), 7.39-7.41 (m,1H) ppm; Anal.Calcd for C13H9N3O:C,69.97; H,4.08; N, 18.88. Found: C, 69.88; H, 3.99; N, 18.95; MS(EI): m/z(%), 223(M+).
RESULT AND DISCUSSION
In an effort to develop more widely applicable methodology, in order to optimize the reaction conditions initially we have studied the efficacy of cobalt(II) chloride by taking catalytic amount of 10 mol% and anthranilamide (1mmol) and benzaldehyde (1.3mmol) in acetonitrile (5mL) as model reaction(3a) refluxed at 70 oC for 5h, the reaction gave corresponding 2-Phenyl-4(3H)-quinazolinone 93% yield. (Table 3, entry 1). In the absence of cobalt(II) chloride even up to 15h no reaction was observed. The model reaction (3a) was performed in various solvents using cobalt(II) chloride as catalyst to identify the best medium for the reaction. A range of solvents such as CHCl3, DMF, THF, DCM, H2O, DMSO, CCl4, and CH3CN were examined and acetonitrile emerged as the solvent of choice in terms of reaction kinetics and product yield (Table 1, entry 7). While comparing the effect of various catalysts on the model reaction (3a) we found that cobalt(II) chloride was more effective than other catalysts tested in terms of isolated yields (Table-2, (3a) entry 7). An optimum amount of 10mol% of cobalt(II) chloride is sufficient to carry forward the reaction. Encouraged by the results obtained for benzaldehyde (3a) we generalized the reaction scope for a number of other structurally divergent aromatic aldehydes, aliphatic aldehydes such as (propanaldehyde, butyraldehyde, and acetaldehyde, Table 3, entry 1j, 1k, 1l) heterocyclic aldehydes (furan-2 aldehyde, thiophene-2 aldehyde Table 3, entry 1f, 1m). In general electron rich counter parts such as hydroxy, methoxy, methyl groups require less reaction time than those of electron withdrawing groups such as (nitro group, halide group) were employed and reacted well to give the corresponding 2-substituted 4(3H)quinazolinones.Table-1
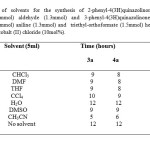 |
Table 1: Screening of solvents for the synthesis of 2-phenyl-4(3H)quinazolinone (3a) from anthranilamide (1mmol) aldehyde (1.3mmol) and 3-phenyl-4(3H)quinazolinones (4a) from anthranilic acid (1mmol) aniline (1.3mmol) and triethyl-orthoformate (1.5mmol) heating at 70oC in the presence of cobalt (II) chloride (10mol%). Click here to View table |
Screening of solvents for the synthesis of 2-phenyl-4(3H)quinazolinone (3a) from anthranilamide (1mmol) aldehyde (1.3mmol) and 3-phenyl-4(3H)quinazolinones (4a) from anthranilic acid (1mmol) aniline (1.3mmol) and triethyl-orthoformate (1.5mmol) heating at 70oC in the presence of cobalt (II) chloride (10mol%).
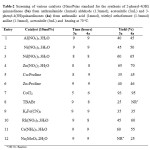 |
Table 2: Screening of various catalysts (10mol%)as standard for the synthesis of 2-phenyl-4(3H) quinazolinone (3a) from anthranilamide (1mmol) aldehyde (1.3mmol), acetonitrile (5mL) and 3-phenyl-4(3H)quinazolinones (4a) from anthranilic acid (1mmol), triethyl orthoformate (1.5mmol) aniline (1.5mmol), acetonitrile (5mL) and heating at 70 oC. Click here to View table |
Encouraged by the results obtained for benzaldehyde (3a) we generalized the reaction scope for a number of other structurally divergent aromatic aldehydes, aliphatic aldehydes such as (propanaldehyde, butyraldehyde, and acetaldehyde, Table 3, entry 1j, 1k, 1l) heterocyclic aldehydes (furan-2 aldehyde, thiophene-2 aldehyde Table 3,
 |
Table 3: Synthesis of 2-substituted -4(3H)quinazolinones by the reaction of aliphatic, aromatic and hetero aromatic aldehydes with anthranilamide, catalyzed by cobalt (II) chloride in acetonitrile at 70 oC. : Click here to View table |
entry 1f, 1m). In general electron rich counter parts such as hydroxy, methoxy, methyl groups require less reaction time than those of electron withdrawing groups such as (nitro group, halide group) were employed and reacted well to give the corresponding 2-substituted 4(3H)quinazolinones. In view of the above results obtained for the synthesis of 2-substituted-4(3H) quinazolinones, we wish to explore the use of cobalt(II) chloride as catalyst for the synthesis of 3-substituted-4(3H)quinazolinone (scheme-2). The treatment of anthranilic acid, triethyl orthoformate (CH(OEt)3), primary amines, in acetonitrile as solvent and cobalt(II) chloride (10mol%) as catalyst, heating at 70 oC for the time specified for each substrate in (Table-4) resulting in the formation of 3-substituted4(3H)quinazolinones. In a similar fashion we studied the efficacy of different catalyst chosen (10mol%) as standard on the model reaction (4a) by taking anthranilic acid, triethyl orthoformate, aniline, acetonitrile, (5mL), heating at 70 oC for 6 hours results in the formation of 3-phenyl-4(3H)quinazolinone in 95% yield (Table-2, (4a) entry 7). The results shown that cobalt(II) chloride is emerged as best catalyst both in terms of reaction time and yields. Model reaction (4a) is screened for the best solvent by taking various solvents and acetonitrile is emerged as the best solvent (Table-1 4a, entry 7). Various 3-substituted 4(3H)quinazolinone were prepared by using structurally varied anilines including pyridine-2-amine (Table-4 entry 2k). All the 2-substituted-4(3H)quinazolinones and 3-substituted-4(3H)quinazolinones are well characterized by spectral analysis and with authentic samples.
 |
Table-4: Synthesis of 3-substituted 4(3H)quinazolinones by the reaction of anthranilic acid (4), HC(OEt)3 (5), aromatic amines, catalyzed by cobalt (II) chloride in acetonitrile at 70oC. Click here to View table |
CONCLUSION
In conclusion we have developed clean and efficient alternative protocols for the synthesis of 2-substituted-4(3H)quinazolinones from anthranilamide, aldehydes and synthesis of 3-substituted-4(3H)quinazolinones from three component reaction of anthranilic acid, triethyl orthoformate, primary amines. The notable feature of these methodologies are by using a mild, inexpensive, easily available, cobalt (II) chloride as catalyst. We believe that this methodology will be a valuable addition for the synthesis of 2-substituted-4(3H)quinazolinones and 3-substituted-4(3H)quinazolinones which are important synthetic interest because of their pharmacological and therapeutic properties such as anti-inflammatory, antiviral, anticancer activities etc.
ACKNOWLEDGEMENT
Dr. Aayesha Nasreen thanks Jazan University, for giving her an opportunity to continue research.
REFERENCES
- Ghorab, M. M.; Farmco, 55, 249 (2000).
- Bradly, D. S.; Tetrahedron Lett., 42, 1851 (2001).
- Kumar, A.; Tyagi, M.; Shrivasthava,V. K.; Indian. J. Chem., 42B, 2142 (2003).
- Brollosy-EI.; Megeed- Abdel, N. R.; Genady, M. F.; Alexandria A. R. ; J. Pharm. Sci. 17(1), 17 (2003).
- Shab, B. R.; Bhatt, J. J.; Patel, H. H.; Undavia, N. K.; Trivedi, P. B.; Desai, N.C. Indian. J. Chem., 34B, 201 (1995).
- Khili, M. A.; Soliman, R.; Furghuli, A. M.; Bekhit, A. A. Arch. Pharm, 27, 327 (1994).
- Shivaram, H. B.; Padmaja, M. T.; Shivnanda, M. K.; Akbarali, P. M. Indian. J. Chem., 37B, 715 (1998).
- Hess, H. J.; Cronin, T. H.; Scriabine, A. J. Med. Chem., 11, 140 (1968).
- Aziza, M. A.; Nassar, M. W.; Abdel Hamide, S. G.; EI-Hakim, A. E.; EL-Azab, A. S. Indian. J. Heterocycl. Chem. 6(1), 25 (1996).
- Pandey, V. K.; Pathak, L. P.; Mishra, S. K. Indian. J. Chem., 44B, 1940 (2005), Pattanaik, J. M.; Paranaik, M.; Bhatta, D. Indian. J. Chem., 37B, 1304 (1998)
- Acharyulu, P.V. R.; Dubey, P. K.; Prasada Reddy, P. V. V.; Suresh,T. ARKIVOC (xi) 104 (2008).
- Rewcastle, G W.; Denny W. A.; Bridges A. J.; Zhou, H.; Cod, R.; McMichael,A.; Fry, D.W, J. Med .Chem., 38, 3482 (1995).
- Rosowsky, A.; Mota, C. E.; Wright, J. E.; Queener, S. F. J.Med.Chem.,37,4522 (1994).
- a) Jiang, J. B.; Hesson, D. P.; Dusak, B. A.; Dexter, D. L.; Kang, G. L.; Hamel, E J .Med.Chem., 33, 1721 (1990) b) Gnana ruba priya, M. International Journal of Pharma and Bio Sciences, 2(1), 295 (2011).
- a) Onaka, T. Tetrahedron Lett. 4387 (1971) b) T. Kametani, T. Loc, C.V.; Higa,T.; Koizumi, M.; Ihara, M.; K. Fukumoto, K.; J. Am. Chem. Soc. 99, 2306 (1977) c) Mori, M. H.; Kobayashi, H.; Kimura, M.; Ban,Y. Heterocycles, 23, 2803 (1977) d) Sauter, F.; Frohlic, J.; Blasl, K.; Gewald, K. Heterocycles, 40, 851 (1985). e) Majo, V.J.; Perumal, P.T.; Tetrhedron Lett. 37, 5015 (1996). f) Prasad, M.; Chen, L.; Repic, O.; Blacklock, T.J.; Synth. Commun. 28, 2125 (1998). g) Connolly, D.J.; Guiry, P.J. Synlett, 1707 (2001) h) Wang, L. Xia, J. F. Qin, F.; Qian, C.; Sun, J. Synthesis, 1241(2003) i) Das, B.; Banerjee, J.; Chem.Lett. 33, 960 (2004).
- a) S. E. Lopez, M. E. Rosales, N. Urdaneta, M. V. Godoy,J. E. Charris, J. Chem. Res., Synop., 6, 258 (2000). b) J. J. Naleway, C. M. J. Fox, D. Robinhold, E. Terpetsching, N. A. Olsen, R. P. Haugland, Tetrahedron Lett. 35, 8569 (1994). c) K. S. Deepthi, D. S. Reddy, P. P.Reddy, P. S. N. Reddy, Indian J. Chem., Sect. B 39, 220 (2000).
- a) Adharvana C. M.; Shobha, D.; Mukkanti, K.; Catalysis Communications, 7, 787 (2006). b) Lingaiah,V. B.; Ezikiel, G. T. Yakaiah; Reddy, V. G.; Rao, P. S. Synlett, 2507 (2006).
- Bi Jing, Xiao.; Li, Z.; Pan, X.; Shi, Y.C.; Journal of the Chinese Chemical Society, 55, 1145 (2008).
- Ighilahriz Karima,; Boutemeur, B.; Chami, F.; Rabia, Cherifa.; Hamdi, M.; Hamdi S. M. Molecules, 13, 779 (2008).
- Raid, J.; Abdel-Jalil.; Voelterband, W.; Saeed, M.; Tetrahedron Letters, 45, 3475 (2004).
- Narasimhulu, M.; Mahesh, K.C.; Srikanth Reddy, T.; Rajesh, K.; Venkateswarlu,Y.; Tetrahedron Letters, 47, 4381 (2006).
- Khosropour, A. R.; Baltorka, I. M.; Ghorbankhani, Hamid. ; Tetrahedron Letters, 47, 3561 (2006).
- Li, F.; Feng, Y.; Meng, Q.; Li, W.; Li, Z.; Wang, Q.; Tao, F.; ARKIVOC, (i ), 40 (2007).
- Adib, M.; Sheikhi,E.; Bijanzadeh, H. R. Synlett, 85 (2012).
- a) Wang G-W.; Miao,C. B.; Kang, H. Bulletin of the chemical society of japan, 79(9), 1426 (2006) b) Durgareddy G. K.; Ravikumar, R.; Ravi, S.; Adapa S. R. J. Chem. Sci. 125, 175 (2013) c) Errede, L. A.; Martinucci, P. D.; McBrady, J. J. J. Org.Chem., 45, 3009 (1980) d) Zentmyer, D. T.; Wagner, E. C.; J. Org.Chem., 14, 967 (1949).
- Rad- Moghadam, K.; Khajavi, S. M. J. Chem. Res., (S) 702 (2006).
- Nasreen, A. Tetrahedron Lett. 54, 3797 (2013).
- Nasreen, A. Asian journal of chemistry, 25, 7535 (2013).
- a) Varala, R.; Nasreen, A.; Ramu, E.; Adapa, S. R. Tetrahedron Lett. 48, 69, (2007) b) Varala, R.; Nasreen, A.; Adapa, S. R. Can. J. Chem., 85, 1, (2007) c) Nasreen, A.; Varala, R.; Adapa, S. R. J. Heterocycl. Chem. 44, 1 (2007).
- a) De, S.K.; Tetrahedron Lett. 45, 1035 (2004) b) De, S.K. Beilstein Journal of Organic Chemistry. http://bjoc.beilstein-journals.org/content/1/1/8, 2005.
- Iqbal, J.; Srivastava, R.R. Tetrahedron Lett. 32, 1663 (1991).
- Velusamy, S.; Borpuzari, S.; Punniyamurthy, T. Tetrahedron, 61, 2001 (2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.









