Biodiesel Production from used Cooking Oil
Y.C.Wong1* and S. Devi1
1Faculty of Agro Based Industry, Universiti Malaysia Kelantan,Jeli Campus, Locked Bag 100, 17600 Jeli, Kelantan, Malaysia.
DOI : http://dx.doi.org/10.13005/ojc/300216
Article Received on :
Article Accepted on :
Article Published : 05 Jun 2014
Production of biodiesel was involved transesterification process that implicated the reaction between used cooking oil and methanol with aid of KOH. The reaction was carried out at 15 min, 30 min, 60 min, 90 min, and 120 min to evaluate the effect of reaction time on the yield of fatty acid methyl ester (FAME). The highest yield of FAME was obtained at reaction time 60 min. Besides that, the yield of fatty acid methyl ester was studied by changing the reaction temperature at 30°C, 60°C, 75°C, 90°C, and 120°C. The maximum yield of fatty acid methyl ester (FAME) was obtained at 60°C.All samples of biodiesel at different reaction time and temperature were contained high amount of bounded glycerol.Biodiesel was successfully reduced 39.2 percent opacities level of smoke released by diesel engine.Biodiesel was less efficient in performance of engine when compared with diesel fuel.
KEYWORDS:transesterification; used cooking oil; yield of FAME; performance of fuel in engine.
Download this article as:| Copy the following to cite this article: Wong Y. C, Devi S. Biodiesel Production from used Cooking Oil. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Wong Y. C, Devi S.Biodiesel Production from used Cooking Oil. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3631 |
Introduction
Biodiesel is an alternative diesel fuel which is environment-friendly, practicably efficient, economically feasible and readily available. It consists of mono alkyl esters of long chain fatty acids derived from vegetable oils or animal fats.Theoretically, biodiesel is called as Fatty Acid Methyl Ester (FAME). The physical and chemical properties of biodiesel are nearly similar to the petroleum diesel in which it operates in diesel engines [1].
Biodiesel is invented through transesterification of vegetable oils with an alcohol in a presence of a catalyst [2]. The catalyst in chemical process of transesterification is to improve the rate of reaction and yield [3].
The objectives of this study are to determinethe effect of reaction time and temperature on the yield of fatty acid methyl ester (FAME) as well as to determine themono-, di- and triolein content in biodieselusing high performance liquid chromatography (HPLC). Besides that, the study was conducted to compare the performance of biodiesel and petroleum diesel by conducting smoke opacity and mileage test.
Materials and Methods
Preparation of Reactants
In this experiment, used cooking oil was experimented. The sample of used cooking oil was collected from a stall selling fried bananas at Melaka.According to the owner of the stall, the cooking oil was used several times for frying before it was ready to be discarded. 5000ml of unwanted used cooking oil was collected from the source and the excessivecomposited fried banana’s scrapswere filtrated by using a clean fine cloth[4].
Titration method was done to estimate the amount of potassium hydroxide (KOH) needed for the process of transesterification to produce fatty acid methyl ester (FAME).
The Effect of Reaction Time on Yield of Fatty Acid Methyl Ester (FAME)
4.4 gram of KOH was used for 500mL of used cooking oil.The KOH was carefully poured into the 100 ml of methanol and stirred well until itcompletely diluted. Then,500 ml of used cooking oil was heated at 60 °C on a hot plate stirrer and stirred slowly using magnetic stirrer to allow uniform distribution of heat. After that, the potassium methoxidewas carefully added into the oil and stirred vigorously for five minutes.
The transesterification reaction took place and methyl ester was separated from glycerol within one hour[5]. The reaction was investigated by varying the reaction time at 15, 30, 60, 90 and 120 minutes. After the transesterification process, glycerol was settled at the bottom of beaker while the methyl ester floated on the top.After the settling, catalyst and soap remained in the methyl ester. Therefore, massive amount of warm water were used to wash away all the contaminants and saturated fatty acids. At the end, the biodiesel was heated at 110 °C for thirty minutes to evaporate water in the fuel [6].
The Yield of used cooking oil wasdeterminedin percentage according to the following Equation 1:
A = B /C × 100% —————————————————————————–Equation 1
whereA is yield percentage of FAME, B is the volume of purified methyl ester (mL) and C is volume of used cooking oil (mL).
The Effect of Reaction Temperature on Yield of Fatty Acid Methyl Ester (FAME)
In this study,4.4 gram of KOH was used for 500mL of used cooking oil. The KOH was poured into the 100 ml of methanol and stirred well until completely diluted. Then, 500 ml of used cooking oil was heated at 60 °C on a hot plate stirrer and stirred slowly using magnetic stirrer to allow uniform distribution of heat. After that, the potassium methoxide was carefully added into the oil and stirred vigorously for five minutes.
The transesterification reaction took place and methyl ester was separated from glycerol within one hour. The effect of temperature on yield of FAME was examined by varying the reaction temperature at 30, 60, 75, 90 and 120°C. After reaction, methyl ester on the top layer of solution was collected and the contaminants in biodiesel were eliminated by washing with water. The small portion of water that remained in biodiesel was evaporated by applying heat at 110°C. The yield of FAME wasdeterminedin percentage according to Equation 1.
Determination of Mono-, Di-, and Tri-olein Content in Biodiesel by Using High Performance Liquid Chromatograph (HPLC)
The composition of bounded glycerol in biodiesel was detected by carrying out qualitative and quantitative analysis using High Performance Liquid Chromatograph (HPLC). 1.5mL of FAME sample was prepared to run into HPLC.The HPLC analysis parameters were determined using the following conditions:column,Hypersil-gold XDB-C-18 (150 × 4.6 mm); oven temperature was 40 °C and 5 µL of the sample was injected into the HPLC system.The mobile phase was 100% of acetonitrile, the flow rate was 1.0 mL/min; and detection was set at a wavelength of 300 nm[7].
Biodiesel Engine Performances
In this study, 2 L of biodiesel was performed in a diesel engine vehicle (Van,Model: MAZDA, Reference Number: MAC 3763). The smoke opacity level and mileage of fuel in engine were analysed.Smoke test was conducted using smoke opacity meter which connected to a computerised strip chart recorder whereas the mileage was determined byallowing fuel to burn completely by idling the engine. Time was recorded when the diesel engine stopped operation. The steps were repeated by performing 2 L of petroleum diesel in same vehicle in order to compare the performance of both fuels.
The fuel mileage was calculated using the following Equation 2
Total distance travelled (m) = Average speed (m/s) x Time taken (s), —————-Equation (2)
where average speed is 80 km/hour
Results and Discussion
The Effect of Reaction Time on Yield of Fatty Acid Methyl Ester (FAME)
Figure 1 showed an increased pattern of graph from reaction time 15 to 60 min. The highest average yield of FAME was recorded about 59 % at reaction time 60 min, while the lowest yield was obtained about 29 % at reaction time 15 min. Nevertheless, there was a clear different in pattern after reaction time 60 min. The yield of FAME decreased slightly about 8 % at 90 min and stayed nearly same at reaction time 120 min.
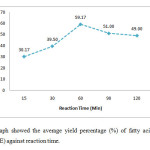 |
Figure 1: The graph showed the average yield percentage (%) of fatty acid methyl acid (FAME) against reaction time. Click here to View Figure |
According to Hayyan et al., (2008), the yield of FAME increases along with reaction time. At 15 min, the rate of reaction was slow due to the mixing stages of methanol into the oil [8]. Hence, it was resulted in lowest yield compared to the yield percentage of other reaction times. The reduction in yield was due to the reversible reaction of transesterification which causes loss of esters and formation of soap. This may also due to the base-catalysedtransesterification which requires less reaction time than acid-catalysedtransesterification [9].
The Effect of Reaction Temperature on Yield of Fatty Acid Methyl Ester (FAME)
The yield of fatty acid methyl ester was investigated by varying reaction temperature at 30°C, 60°C, 75°C, 90°C, and 120°C. According to the results summarised in Figure 2, the reaction temperature were showed a significant effect on yield of free fatty acid to methyl ester. In this study, the lowest yield of was around 30 % at reaction temperature 30°C. The maximum yield of FAME was obtained at 60°C. The yield of FAME drastically dropped due to the increased in temperature up to 120°C.
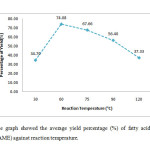 |
Figure 2: The graph showed the average yield percentage (%) of fatty acid methyl acid (FAME) against reaction temperature. Click here to View Figure |
A higher reaction temperature can increase the rate of reaction through reduction in viscosity of used cooking oils. Since the boiling point of methanol is 64.7°C, the elevation of temperature may results in evaporation of the alcohol. Thus, it was clearly lowered the yield of FAME. Studies by Leung &Guo, (2006) showed that the temperature above the optimal range will lead to reduction in biodiesel yield due to the saponification of triglycerides [10]. According to Itodo(2010), the optimal reaction temperature is dependent on type of feedstock used. The maximum range of optimal reaction temperature is obtained at ranging from 60°C to 80°C. Further increase in temperature will decrease the yield [11].
Determination of Mono-, Di-, and Tri-olein Content in Biodiesel by Using High Performance Liquid Chromatograph (HPLC)
Purity of biodiesel was investigated by qualitative and quantitative analysis using high performance liquid chromatograph (HPLC). The important aspect in biodiesel production is to determine the completeness of transesterification process.Berchmanset al., (2007)emphasised that the unconverted triglycerides, diglycerides and monoglycerides will be remained in reaction mixture when the reaction was incompleted. Each of these compounds still contains a glycerol molecule that has not been eliminated [12]. This portion of glycerol is called as bound glycerol.
Qualitative Analysis
Mono-, di- and triglycerides contents in biodiesel was identified from calibration curves according to standards (Monoolein-solution OEKANAL® 5000 µg/mL in pyridine, 1,3- Diolein-solution OEKANAL® 5000 µg/mL in pyridine and Triolein-solution OEKANAL® 5000 µg/mL in pyridine) of ASTM D-6584. All the standards and samples of biodiesel were analysed in HPLC under same conditions.
The peak of standards mono-, di- and triolein were eluted at retention time 3.372, 3.380 and 3.409 respectively. The peaks were appeared significantly closer to each other. However, monolein was eluted at shortest retention time and followed by diolein and triolein in ordered series. Identification peak of mono-, di-, and triolein were based on the comparison of calibration peaks of samples with standard peak at a specific retention time. Corresponding to studies of Kromidas (2000), the evaluation of chromatograms was depending on the peak resolution and retention time. Peak resolution refers to the value that measured by the distance between two peaks at their base [13].
Based from tabulated results in Table 1, samples with different reaction time were analysed to identify quality of each samples of biodiesel. The peak of monoolein in samples 15 min, 30 min, 60 min, 90 min and 120 min were identified at the range 3.502 to 3.517 min, where it deviated 0.1 min retention time of the standard. The chromatogram of the same samples was used to compare the other standards to identify calibration peak of diolein and triolein. Theidentification peak of diolein in the same sample was presented at 3.659 to 3.821 retention time. The variation in the retention time was typically 0.2 to 0.4 min. Next, the identification peak of triolein was obtained at 3.813 to 4.006 min, where it deviated 0.4 to 0.5 min retention time of the standard.
Table 1: The retention time of calibration peaks formed by quality analysis of biodiesel samples with different reaction time and standards of mono-olein, di-olein and tri-olein using HPLC.
| Sample | ||||||
| Component | Standard | 15min | 30 min | 60 min | 90 min | 120min |
|
Mono-olein |
3.372 |
3.502 |
3.507 |
3.505 |
3.517 |
3.504 |
|
Di-olein |
3.380 |
3.659 |
3.818 |
3.818 |
3.821 |
3.686 |
|
Tri-olein |
3.409 |
3.813 |
4.002 |
4.006 |
4.004 |
3.853 |
Throughout the observation, all samples of biodiesel at different reaction time were contained high by-products of bounded glycerol (monoglycerides, diglycerides at triglycerides). Therefore, it reduced the quality of biodiesel and the purity of fuel was affected. McCormick & Westbrook (2007)emphasised that glycerol should be removed from the FAMEs because it can cause undesirable effects on diesel engine [14].
According to Table 2, samples with different reaction temperature were analysed to identify quality of each samples of biodiesel. The peak of monoolein in samples 30°C, 60°C, 75°C and 120°C were identified at the range 3.502 to 3.511 min, where it deviated 1.0 min retention time of the standard. The chromatogram of the same samples was used to compare the other standards to identify calibration peak of diolein and triolein. The identification peak of diolein in the same sample was presented at 3.818 to 3.824 retention time. The variation in the retention time was 0.4 min. Next, the identification peak of triolein was obtained at 4.006 to 4.012 min, where it deviated 0.5 min retention time of the standard.
Table 2: The retention time of peaks formed by quality analysis of samples with different reaction temperature and standards of mono-olein, di-olein and triolein using HPLC.
|
Sample |
||||||
| Component | Standard |
30°C |
60°C |
75°C |
90°C |
120°C |
|
Mono-olein |
3.372 |
3.511 |
3.502 |
3.502 |
3.509 |
3.505 |
|
Di-olein |
3.380 |
3.824 |
3.822 |
3.821 |
3.820 |
3.818 |
|
Tri-olein |
3.409 |
4.008 |
4.012 |
4.010 |
4.008 |
4.006 |
All samples of biodiesel at different reaction temperature were contained high by-products of bounded glycerol (monoglycerides, diglycerides at triglycerides) which may due to the insufficient of purification techniques. Fernando et al., (2007) describes that glycerol is a potential reason for biodiesel instability. Hence, high amount of bounded glycerol could affect the performance of oil due to the instability [15].
Quantitative Analysis
The concentration of monoolein, diolein and tri-olein in each samples were identified by creating a calibration curve of standards.Referring to the tabulated data in Table 3, Table 4 and Table 5, the highest concentration of monoolein, diolein and triolein were 76.42, 222.44 and 62.08 percent respectively in sample of 120 min. Both monoolein and diolein concentration were least at reaction time 90 min, while the lowest concentration of triolein was at reaction time 30 min.
Table 3: The concentration of monoolein in samples with different reaction time which calculated from standard curve equation,y =95848x +280540.
|
Samples |
Area, y |
Concentration, Wt (%), x |
|
15 |
3455595 |
33.13 |
|
30 |
7231553 |
72.52 |
|
60 |
3650001 |
35.15 |
|
90 |
3116511 |
29.59 |
|
120 |
7605083 |
76.42 |
Table 4: The concentration of diolein in samples with different reaction time which calculated from standard curve equation, y = 14025x + 72238.
|
Samples |
Area, y |
Concentration, Wt (%), x |
|
15 |
1359412 |
91.77 |
|
30 |
542914 |
33.56 |
|
60 |
598473 |
37.52 |
|
90 |
523515 |
32.17 |
|
120 |
3192096 |
222.44 |
Table 5: The concentration of triolein in samples with different reaction time which calculated from standard curve equation, y =130004x + 229927.
|
Samples |
Area, y |
Concentration, Wt (%), x |
|
15 |
489275 |
19.94 |
|
30 |
2597257 |
18.20 |
|
60 |
2939861 |
20.84 |
|
90 |
2727720 |
19.21 |
|
120 |
8301078 |
62.08 |
Studies of Bautista (2009) &Encinar (2010) showed that reaction time more than 90 minutes will be resulted in reversible reaction which triggers the formation of reactant. Hence, the total glycerol and FAME that produced after reaction might be converted back into monoglycerides, diglycerides and triglycerides [16,17]. Monoolein, diolein and triolein contents in biodiesel were increased at longer reaction time.
Based on Table 6, Table 7 and Table 8, the highest concentration of monoolein and triolein were 41.88 and 25.78 percent in sample of 60 °C, whereas diolein was highest about 30 percent in sample of 90 °C. The lowest concentration of monoolein, diolein, and triolein were recorded at reaction temperature 30 °C, 60 °C and 90 °C respectively.
Table 6: The concentration of monoolein in samples with different reaction temperature which calculated from standard curve equation,y =95848x + 280540.
|
Samples |
Area, y |
Concentration,Wt (%), x |
|
30 |
3440966 |
32.97 |
|
60 |
4295515 |
41.88 |
|
75 |
4011433 |
38.92 |
|
90 |
3522987 |
33.82 |
|
120 |
4059952 |
39.43 |
Table 7: The concentration of di-olein in samples with different reaction temperature which calculated from standard curve equation, y = 14025x + 72238.
|
Samples |
Area, y |
Concentration, Wt (%), x |
|
30 |
439940 |
26.21 |
|
60 |
407568 |
23.90 |
|
75 |
441501 |
26.32 |
|
90 |
512115 |
31.36 |
|
120 |
492791 |
29.98 |
Table 8: The concentration of tri-olein in samples with different reaction temperature which calculated from standard curve equation, y =130004x + 229927.
|
Samples |
Area, y |
Concentration, Wt (%), x |
|
30 |
3581902 |
25.78 |
|
60 |
4284170 |
31.18 |
|
75 |
3821286 |
27.62 |
|
90 |
3072587 |
21.86 |
|
120 |
4054075 |
29.41 |
The formation of by-product in the samples was mainly due to reversible reaction that triggers saponification. The reaction is driven towards the reactant side as the temperature increases above the optimal condition.
The overall results showed that the FAME produced at different reaction time and temperature contained high concentration of unreacted partial glycerides such as monoglycerides, diglycerides and triglycerides. The concentration of total glycerine in FAME of all samples was exceeded the specification limits that given by ASTM D6751. According to ASTM D6751, the concentration of total glycerine should not exceed 0.240 Wt%.
Feedstock of biodiesel could be the major influence of saponification. Overheating of used cooking decreases the quality of oil by oxidation, hydrolysis and polymerization [18]. Hydrolysis of triglycerides in feedstock forms carboxylic acid which might be reacted with KOH to form soap. Washing of biodiesel with water became more difficult due to emulsion formation. Emulsification was resulted due to the large amount of soap formation in samples. Therefore, there are some by-products remained unwashed in the FAME samples [19].
Biodiesel Engine Performances
The efficiency and consumption test of biodiesel fuel was tested to identify its possible environmental and long term economic benefits compared to petroleum diesel. The meter able to detect the smoke density that emitted from diesel engine by measure the amount of light blocked the vision.
Based on the summarised results in Figure 3, biodiesel was successfully reduced 39.2 percent opacities level of smoke released by diesel engine that performed with petroleum diesel. Performance of diesel in engine was resulted in high altitude of smoke opacities compared to biodiesel.
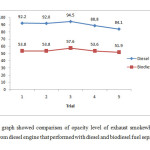 |
Figure 3: The graph showed comparison of opacity level of exhaust smokewhich released from diesel engine that performed with diesel and biodiesel fuel separately. Click here to View Figure |
According to Pope (1995), smoke opacity is implicit scale of soot content in exhaust gases [20]. The high emission of particulate matter (PM) was apparently increased the smoke opacities. Agudeloet al., (2010) reported in his studies that biodiesel were showed a significant positive influence on smoke emission [21].
Many reports showed that incomplete combustion of fuel in engine could cause in emission of particulate matter to the environment due to the lack of oxygen. The significant decrease of
Table 9: Time taken for the complete engine performance using different types of fuel.
|
Fuel Sample |
Completion Time (Min) |
|
Biodiesel |
12.00 |
|
Diesel |
15.00 |
smoke opacity for biodiesel performance is mainly attributed to the reduction of aromatics and existence of oxygen in ester molecules [22]. Ester fuel with higher oxygen content contributes more oxygen for the complete combustion and soot oxidation. Therefore, the gases emitted from the biodiesel performance engine attained lowest opacity altitude.
The efficiency of both biodiesel and diesel was also tested by performing the fuels in a diesel engine Van (engine ability, 2184 S.P). Mileage of each types of fuel was determined by substituting completion time which tabulated in Table 9 into Equation 2. The calculated data was recorded in Table 10.
Table 10: The fuel mileage in diesel engine using different types of fuel.
|
Fuel Sample |
Distance Travelled (Km) |
|
Biodiesel |
15.99 |
|
Diesel |
19.99 |
Engine that performed with biodiesel showed the shorter distance travelled compared to diesel performance in the same engine. There was 4 km difference in mileage of the both fuels. The results indicated that biodiesel was less efficient in performance of engine when compared with diesel performance. Jain (2010) reported that B100 increased the Brake Specific Fuel Consumption (BSFC) (gm/kWh) due to its lower power than diesel [23]. According to Jason (2010), engine power decreases when performed with biodiesel due to the reduction in heating value of biodiesel compared to diesel which resulted in high consumption of biodiesel fuel [24].
Conclusion
Effect of reaction time on yield of FAME was investigated in this study. The amount of yield was varied at different reaction time. The lowest yield was recorded at reaction time 15 min, while the highest yield was reached at reaction time 60 min. Short reaction time will be resulted in incomplete transesterification process as the time was insufficient for the used cooking oil to blend completely with methanol.
The yield of FAME was also affected by the reaction temperature. The difference between the highest and lowest yield of FAME was about 28 percent. High temperature was increased the rate of reaction. Maximum yield of FAME can be obtained at optimum reaction time and temperature. Lowering in yield was due to the reaction above optimum temperature and time. From the study, the optimum results were obtained at reaction time 60 min and reaction temperature 60°C respectively.
Besides that, qualitative and quantitative analysis was done to identify the presence of impurities of bonded glycerol in biodiesel samples after the process of purification. The result showed that all the samples of biodiesel that produced at different time and temperature had high level of by-products of glycerine. According to the specification limits that recommended by ASTM D6751 methods, total glycerine should not exceed 0.240 Wt% in order to obtain a pure biodiesel. All samples of biodiesel were exceeded these recommended limits. The frequent performance of the fuel in diesel engine could deposits the impurities in storage tank, as well as clogging the fuel filters.
The efficiency of biodiesel and petroleum diesel was also investigated by performing both fuels in unmodified diesel engine. Biodiesel was clearly showed a positive influence on smoke emission. Therefore, biodiesel gives environmental benefits compared to petroleum diesel. Besides that, the fuel efficiency in diesel engine was determined by comparing diesel and biodiesel performance in the term of distance travelled. Diesel that performed with biodiesel travelled shorter distance compared to performance of diesel. Hence, performance of biodiesel is less efficient than petroleum diesel.
Acknowledgement
The author would like to give a sincere gratitude to Universiti Malaysia Kelantan (UMK), for their financial supports and laboratory facilities provided in completing this study.
References
- Srivastava, A., Prasad, R. (2000). Triglycerides-based diesel fuels. Renewable Sustainable Energy: 111-133.
- Chris Collins (2007). Implementing Phytoremediation of Petroleum Hydrocarbons, Methods in Biotechnology.Humana Press, 23: 99–108.
- Knothe G. and K.R. Steidley (2005). Kinematic viscosity of biodiesel fuel components. Influence of compound structure and comparison to petrodiesel fuel components. Fuel Processing Technology, 86: 1059-1065.
- Zhang Y., Dube M. A., Mclean D. D. andKates M. (2003). Biodiesel Production from Waste Cooking Oil.Process Design and Technological Assessment.Bioresource Technology, 89: 1-16.
- Canakci M. and Van Gerpen J. (2003). A Pilot Plant to Produce Biodiesel from High Free Fatty Acid Feedstocks.American Society of Agricultural and Biologi-cal Engineers, 46: 945-954.
- Gerpen, J. V.(2005). Biodiesel processing and production.Fuel Processing Technology, 86: 1097-1107.
- Myller S. Carvalho, Márcio A. Mendonça, David M. M. Pinho, Inês S. Resck, Paulo A. Z. Suarez (2012). Chromatographic analyses of fatty acid methyl esters by HPLC-UV and GC-FID, Journal of Brazil Chemistry Society, 23: 4.
- Hayyan A., Alam M.Z., Kabbashi N.A., Mirghani M.E.S., Siran Y.M., HakimiN.I.N.M. (2008). Pretreatment of sludge palm oil (SPO) for biodiesel production by esterification, Proceedings of Symposium of Malaysian Chemical Engineers (SOMchE), 2: 485-490.
- Georgogianni, K.G, Katsoulidis A.K., Pomonis P.J., Manos G. and KontominasM.G. (2009). Transesterification of rapeseeds oil for the production of biodiesel using homogeneous and heterogeneous catalysis. Fuel Processing Technology, 90: 1016-1022
- Leung, D. Y. C.; Guo, Y. (2006). Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Processing Technology, 87 (10): 883–890
- Itodo IN, Oseni, MI, Wergba C. (2010). A comparative study of the properties and yield of biodiesel from Soy and groundnut oils.Solar Energy, 21:124-127.
- Berchmans, HannyJohanes and Hirata, Shizuko (2007). Biodiesel Production from Crude JatrophaCurcas L. Seed Oil with a High Content of Free Fatty Acids.Bioresource Technology, 99: 1716 – 1721.
- Kromidas, S. (2000). In Practical Problem Solving in HPLC; Wiley-VCH: Weinheim, Germany.
- McCormick, R.L. and WestbrookS.R.(2007). “Biodiesel and Biodiesel Blends,”.ASTM Standardization News, 35(4): 28–31.
- Fernando S, Karra P, Hernandez R &Jha S K. (2007). Effect of incompletely converted soybean oil on biodiesel quality.Energy, 32: 844-851.
- Bautista Luis Fernando, Gemma Vicente, Rosalia Rodriguez, Maria Pacheco (2009).Optimization of FAME production from waste cooking oil for biodiesel use.Biomass and Bioenergy, 33: 862-872.
- Encinar J. M., Gonzalez J. F., Martinez G., Sanchez N. and Gonzalez C. G. (2010). Synthesis and Characterization of biodiesel obtained from castor oil transesterification. International Conference on Renewable Ener-gies and Power Quality (ICREPQ’11). Las Palmas de Gran Canaria (Spain).
- Naz, S., Siddiqi, R., Sheikh, H. and Sayeed, S.A. (2005). Deterioration of olive, corn and soybean oils due to air, light, heatand deep-frying.Food Research International, 38: 127-134.
- Azcan, N., Danisman, A. (2007) Alkali catalyzed transesterification of cottonseed oil by microwave irradiation. Fuel, 86: 2639-2644.
- Pope 3rd CA, Dockery DW. (1995). Health effects of fine particulate air pollution: lines that connect. Journal of the Air & Waste Management Association, 56: 709–42.
- Agudelo J, Benjumea P, Villegas AP. (2010). Evaluation of nitrogen oxide emissions and smoke opacity in a HSDI diesel engine fuelled with palm oil biodiesel. RevistaFacultad de Ingenierı´a Universidad de Antioquia, 42: 62–71.
- Anand K., Sharma R.P, Mehta P.S. (2011). Experimental investigations on combustion, performance and emission characteristics of neat karanji biodiesel and its methanol blend in diesel engine. Biomass and Bioenergy, 35: 533-54.
- Jain S., Sharma M.P. (2010). Biodiesel Production.Renewable and Sustainable Energy Reviews, 14: 667–678.
- Jason Y.W. Lai, Kuang C. Lin, Angela Violi (2011). Biodiesel combustion: Advances in chemical kinetic modelling. Progress in Energy and Combustion Science, 37: 1-14.

This work is licensed under a Creative Commons Attribution 4.0 International License.









