An Eco-Friendly System for Oximation of Organic Carbonyl Compounds Under Microwave Irradiation
Hana Batmani and Davood Setamdideh*
Department of Chemistry, College of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, Iran.
DOI : http://dx.doi.org/10.13005/ojc/300241
Article Received on :
Article Accepted on :
Article Published : 28 Jun 2014
The oximation of a variety of organic carbonyl compounds was efficiently carried out with NH2OH·HCl under microwave irradiation. The reactions were performed in water or water-ethanol as green solvents to give Z-aldoxime isomers from the corresponding aldehydes and E-ketoxime isomers from the corresponding ketones in a perfect selectively with excellent yields.
KEYWORDS:Oximes; Z-aldoximes; E-acetophenone oximes; H2NOH.HCl; microwave irradiation
Download this article as:| Copy the following to cite this article: Batmani H, Setamdideh D. H.An Eco-Friendly System for Oximation of Organic Carbonyl Compounds Under Microwave Irradiation. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Batmani H, Setamdideh D. H.An Eco-Friendly System for Oximation of Organic Carbonyl Compounds Under Microwave Irradiation. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=4037 |
Introduction
Microwave irradiation as an unconventional energy source has been widely used to carry out many kinds of chemical reactions. Many reviews and papers have demonstrated its importance. It is now clear that the microwave dielectric heating effect uses the ability of liquids to transform electromagnetic energy into heat and so drive chemical reactions effectively and quickly1. This in situ mode of energy conversion has attracted much attentions of chemists and has resulted in the development of many techniques to perform microwave-assisted organic reactions using domestic microwave ovens. On the other hand, the use of large amounts of conventional volatile solvents required to conduct a chemical reaction creates economic and ecological concerns. Consequently replacement of volatile organic solvents from reaction medium has been a major emphasis of green chemistry2. So, the use of water as a green solvent can be the best medium of choice to perform chemical reactions. In the last decades, a large number of publications have demonstrated the value of performing chemical reactions in water or aqueous media 3a-b. We have reported a fast and efficient method for the reduction of varieties of carbonyl compounds such as aldehydes, ketones, conjugated aldehydes and ketones, α-diketones and acyloins to their corresponding alcohols under microwave irradiation in water as green solvent3c.
Recently, the oximation methods has been reviewed 4. The lack of information for systematic the oximation of carbonyl compounds in water under microwave irradiation and our ongoing attentions to the development of modified methods in organic synthesis 5-23 encouraged us to investigate this transformation with NH2OH·HCl under microwave irradiation in water as a green and good solvent for transferring electromagnetic energy into heat and so driving an oximation reaction effectively. Herein, we describe a fast and efficient method for the oximation of varieties of carbonyl compounds such as aldehydes, ketones to the corresponding oximes with NH2OH·HCl/H2O/MWI system.
Results and Discussions
The oximation of unsymmetrical carbonyl compounds usually give a mixture of the two geometrical isomers (Z and E) of their corresponding oximes which have different physical properties and biological activities24. Thus, there is considerable interest in finding more selective methods for oximes synthesis. We now report a simple and efficient method for the preparation of oximes from their corresponding carbonyl compounds with NH2OH·HCl/H2O/Microwave irradiation system.
In order to determine an appropriate reaction conditions for the oximation of aldehydes and ketones a model study was carried out on the oximation of bezaldehyde and acetophene. The results showed that using NH2OH.HCl (1mmol) in H2O (5 ml) and 30% power amplitude of microwave oven (300 W) was the best for the oximation of benzaldehyde. The reaction was completed in 90 sec with excellent yield (95%). The results show that the use of 1 molar equivalents of NH2OH.HCl is sufficient and no other additives are required for this conversion. In order to evaluate the generality of the process a variety of aldehydes were ground with hydroxylamine hydrochloride under microwave irradiation in water. In this approach, the corresponding Z-aldoximes were obtained in quantitative yield. The general reaction has been shown in scheme 1 and the results have been reported in table1 (entries 1-
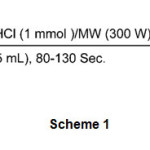 |
Scheme 1 Click here to View Scheme |
The reaction of different aromatic aldehydes gave Z-aldoximes in excellent yields and stereoselectivity. The purity of the products was determined by 1H-NMR which showed the exclusive formation of the corresponding Z-aldoximes. The Z-stereochemistry of the products was determined from the 1H-chemical shift 4 of the C(H)=N group which appeared around 8-8.5 ppm as a singlet whereas the 1H-chemical shift of the C(H)=N group for E-aldoximes appears in 7.30 and 7.60 ppm. In all the 1H-NMR spectra (CDCl3, 25 ºC) by comparison of 1H-NMR of these isomers, we have observed the C(H)=N signal in 8.10 -8.49 ppm. The 1H-chemical shift of the C(H)=N groups appears for (Z)-benzaldehyde oxime in δ 8.18 ppm, (Z)-4- bromo benzaldehyde oxime in δ 8.10 ppm, (Z)- N, N-dimethylbenzaldehyde oxime in δ 8.07 ppm, (Z)-4-methyl benzal dehyde oxime in δ 8.15 ppm, (Z)-3-methyl benzaldehyde oxime in δ 8.15 ppm, (Z)-2-methoxy benzalde hyde oxime in δ 8.49 ppm and (Z)-4-methoxy benzalde hyde oxime in δ 8.11 ppm.
The oximation of ketones was also performed well by NH2OH·HCl/H2O-EtOH/Microwave irradiation system but due to the lower reactivity of ketones relative to aldehydes, the oximation requires higher molar amounts of NH2OH·HCl (2 mmol) vs. 1mmol of the substrates. E-acetophenone oximes (table 1, entries 8-11) were also obtained in high to excellent yields as shown in scheme 2.
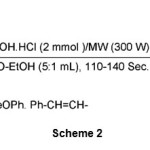 |
Scheme2Click here to View Scheme |
The E-stereochemistry of acetophenone oxime derivatives was determined from the 1H-chemical shift of the CH3 group which appeared around 2.3 ppm as a singlet whereas the 1H-chemical shift of the CH3 group for Z-ketoximes appeared around 2.6 ppm. In all the 1H-NMR spectra (CDCl3, 25 ºC) by comparison of 1H-NMR of these isomers 4, we have observed the CH3 signal in 2.30-2.34 ppm. The 1H-chemical shift of the CH3 groups appears for (E)-benzalacetone oxime in δ 2.34 ppm, (E)-4-methylacetophenone oxime in δ 2.38 ppm, (E)-4-methoxyacetophenone oxime in δ 2.30 ppm and (E)-benzalacetone oxime (as α,β-unsaturated ) in δ 2.18. The Symmetric ketones (table 1, entries 12-14). i.e. Benzophenone, 9H-fluoren-9-one and cyclohexanone were ground with hydroxylamine hydrochloride under microwave irradiation in water to their corresponding ketoximes in quantitative yields.
In order to show chemoselectivity of the presented oximation system a mixture of one equivalents of benzaldehyde and one equivalents of acetophenone was treated with NH2OH.HCl (1mmol) under microwave irradiation in water. The oximation of aldehyde with respect to ketone was not satisfactory. In the most cases the selectivity ratios were not excellent. Therefore this methodology could not be used selectively for the preparation of aldoximes of compounds that contain both aldehyde and ketone functional groups.
Experimental
All microwave assisted reactions were carried out in a Yusch household microwave oven (1000 W). The instrument was modified for laboratory applications with an external reflux condenser. All substrates and reagents were purchased from commercially sources with the best quality. IR and 1H NMR spectra were recorded on Perkin-Elmer FT-IR RXI and 300 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples (melting points). All yields referred to isolated pure products. The purity of products was determined by TLC and 1H NMR. Also, reactions were monitored by TLCs utilizing plates cut from silica gel 60 F254 aluminum sheets.
A typical procedure for the oximation of aldehydes with NH2OH·HCl/H2O/Microwave irradiation system
In a round-bottomed flask (10 mL) a mixture of benzaldehyde (0.106 g, l mmol) in water (5 mL) was prepared. NH2OH·HCl (0.07 g, 1 mmol) was added. After fitting the flask to the external condenser at the inside of the oven the mixture was irradiated with a microwave oven (30% power amplitude ≈ 300 W) for 90 sec. The progress of the reaction was monitored by TLC (eluent:CCl4/Et2O:5/2). After completion of the reaction, the precipitate Z-benzalde oxime (0.115 g, 95 % yield, table1, entry 1) was filtered and washed with water and air-dried without further purification.
 |
Table1: Oximation of Carbonyl Compounds by NH2OH.HCl/EtOH/H2O under Microwave Irradiation (300 W) Click here to View table |
A typical procedure for the oximation of ketones with NH2OH·HCl/H2O/Microwave irradiation system
In a round-bottomed flask (10 mL) a solution of acetophenone (0.120 g, l mmol) in water-ethanol (96%) (5:1 mL) was prepared. NH2OH·HCl (0.14 g, 2 mmol) and was added. After fitting the flask to the external condenser at the inside of the oven the mixture was irradiated with a microwave oven (30% power amplitude ≈ 300 W) for 130 sec. The progress of the reaction was monitored by TLC (eluent:CCl4/Et2O:5/2). After completion of the reaction the precipitate i.e.E-acetophenone oxime (0.126 g, 93% yield, table 1, entry 8) was filtered and washed with water and air-dried without further purification.
Conclusion
In conclusion, the oximation of a variety of carbonyl compounds such as aldehydes, ketones was carried out efficiently with NH2OH·HCl/H2O/Microwave irradiation system. The reactions were performed in water and water-ethanol to give Z-aldoximation isomers of aldehydes and E-oximaton of ketones in a perfect selectively. The oximation of aldehydes over ketones has not been accomplished successfully by this system. Also, this oximation system has the easily worked up. Therefore, this new protocol for the oximation of carbonyl compounds could be a useful addition to the present methodologies.
Acknowledgements
The authors gratefully appreciated the financial support of this work by the research council of Islamic Azad University branch of Mahabad.
References
- (a) Loupy, A. Microwaves in Organic Synthesis; JohnWiley & Sons, New York, (2002); (b) Kidwai, M. Pure Appl. Chem., 73: 147(2001).
- (a) Nagendrappa, G. Resonance., 59 (2002); (b) Varma, R. S.; Namboodiri, V. V. Pure Appl. Chem., 73: 1309 (2001); (c) Varma, R. S. Green Chem., 43 (1999).
- (a) Grieco, P. A. Organic Synthesis in Water; Blackie Academic & Professional, New York, (1998); (b) Adams, D. J.; Dyson, P. J.; Tavener, S. J. Chemistry in Alternative Reaction Media; John Wiley & Sons, New York, (2003); (c) Zeynizadeh, B.; Setamdideh, D. J. Chin. Chem. Soc., 52: 1179 (2005).
- Setamdideh, D.; Khezri, B.; Esmaeilzadeh, S. J. Chin. Chem. Soc., 59: 1119 (2012).
- Setamdideh, D.; Karimi, Z.; Rahimi, F. Orient. J. Chem., 27: 1621(2011).
- Setamdideh, D.; Khezri, B.; Mollapour, M. Orient. J. Chem., 27: 991(2011).
- Setamdideh, D.; Khezri, B.; Rahmatollahzadeh, M.;Aliporamjad, A. Asian J. Chem. 24: 3591(2012).
- Setamdideh, D.; Rafig, M. E-J. Chem., 9: 2345 (2012).
- Setamdideh, D.; Rahmatollahzadeh, M. J. Mex. Chem. Soc., 56 :169 (2012).
- Setamdideh, D.; Ghahremani, S. S. Afr. J. Chem., 65: 91(2012).=
- Setamdideh, D.; Hasani, S.; Noori, S. J. Chin. Chem. Soc., 60: 1267 (2013).
- Pourhanafi, S.; Setamdideh, D.; Khezri, B. Orient. J. Chem. 29: 709 (2013).
- Setamdideh, D.; Khezri, B.; Rahmatollahzadeh, M. J. Serb. Chem. Soc., 79: 1 (2013).
- Mohamadi, M.; Setamdideh, D.; Khezri, B. Org. Chem. Inter., doi:10.1155/2013/127585 (2013).
- Latifi Mmaghani, E.; Setamdideh, D. Orient. J. Chem. 29: 953 (2013).
- Kamari, R.; Setamdideh, D. Orient. J. Chem. 29: 497 (2013).
- Setamdideh, D.; Khaledi, L. S. Afr. J. Chem., 66: 150 (2013).
- Setamdideh, D.; Karimi, Z.; Alipouramjad, A. J. Chin. Chem. Soc., 60: 590 (2013).
- Setamdideh, D.; Sepehraddin, F. J. Mex. Chem. Soc., 58: 22 (2014).
- Azizi Asl, P.; Setamdideh, D. J. Chin. Chem. Soc., 61: (2014). doi:10.1002/jccs.201400049
- Setamdideh, D.; Minaee, M. Orient. J. Chem. 30: 359 (2014).
- Arefi, H.; Setamdideh, D. Orient. J. Chem. 30: 299 (2014).
- Taie Hasanloie, S.; Setamdideh, D. Orient. J. Chem. 30: 341 (2014).
- Xu, W; Wang, J.; Liu, C.; Chen, C. L. J. Chin. Chem. Soc. 51: 1259 (2004).

This work is licensed under a Creative Commons Attribution 4.0 International License.









