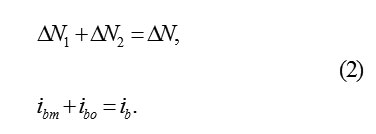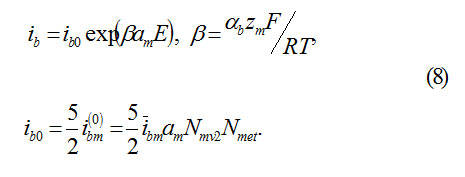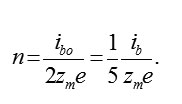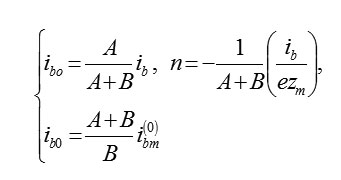A Physical Model of the Interphase Boundary of the Passivating Layer on the Metal in the Electrolyte Solutions
Saif A. Mouhammad , Ayman F. Amer
Department of Physics, Taif university, Saudi Arabia
DOI : http://dx.doi.org/10.13005/ojc/300235
Article Received on :
Article Accepted on :
Article Published : 11 Apr 2014
The model of processes on the interphase boundary metal- passivating layer is proposed. The balance of ions upon the formation of the cells of oxide is examined. The velocity expressions of these processes are obtained.
KEYWORDS:The mechanism of processes A Physical Model of the Interphase
Download this article as:| Copy the following to cite this article: Mouhammad A. S, Amer. A. F. A Physical Model of the Interphase Boundary of the Passivating Layer on the Metal in the Electrolyte Solutions. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Mouhammad A. S, Amer. A. F. A Physical Model of the Interphase Boundary of the Passivating Layer on the Metal in the Electrolyte Solutions. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=2883 |
Introduction
The mechanism of processes on the interphase boundary of the passivating oxide layer PL has a fundamental value, whereas, the properties of the passivating layer are determined by them.In this paper a consistent model of the boundary metal-oxide MO is presented.
General stationary laws
On the interphase boundary of metal with PL, the continuous dissolutionof metal occurs.it disappears consecutively monolayer after a monolayer and the cells of oxide occupy its place. if at time the layer of metal of thickness disappears, then the plane MO displaces in its side with the speed , equals
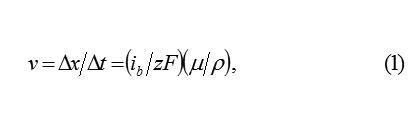
where ib– total dissolution current; μp- the molar mass and density.The thickness of the oxide layerΔok substituting ΔM for the same timeΔt , is also equal Δx . let us designate a quantity of ions in the initial ΔM layer of metal and its substituted layer of oxide Δok by and ΔN1 respectively. It is obvious, that ΔN1 < ΔN, since the specific volumes, according to the metal ion, in ΔM and Δok are different. Consequently, only ions in ΔN1 a layer ΔM in volved in the formation of oxide . Δok The remaining part, equal , ΔN2 = ΔN ΔN1, considered to be excess and must leave into the solution through the boundary MO and PL. Therefore, the balance of space in the layer of metal and the oxide is observed.considered to be excess and must leave into the solution through the boundary MO and PL. Therefore, the balance of space in the layer of metal ΔM and the oxide Δok is observed.Thus the surface MO serves as the source of ions MZ+ok , and in the passivating layer, their intensive transfer by the mechanism of hopping migration in the strong electric field should be occurred. Accordingly, the total current of the dissolution of metal is divided into the transfer current of the ions through the boundary MO in PL and the current of oxide formation on this boundary (subscripts indicate: b- boundary, m- metal, o- oxygen; the current ibo determines a quantity of metal ions, which in 1cm3 every second are connected in the layer ΔM with oxygen), such as
The subsequent analysis confirms this conclusion. The total current ib must be equal
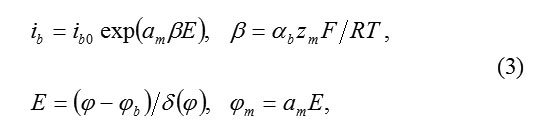
Where am – interatomic distance in the cationic sublattice, αb transfer coefficient on the boundary MO.
Equation(3) assumes, that an ion M,passing into the state of anion MZ+ok must overcome a potential jump ΦM= αME. Thus, the barrier of transition decreases to ΔWM= αbzMΦM The constant ib0 of transition speed is determined from the independent temperature measurements ( for Fe, for example, )ibo ˜10-10A/cm2
The total current ib is the fundamental characteristic of stationary processes on the interphase boundary MO, and the micro scheme of the formation of cells M2O3 are ambiguous. They must consider a difference between the interatomic distances and the angular directions in the lattices of metal and oxide, and require the use of probabilistic methods for describing regrouping particles.
Further, it is interesting to detail a physical situation on a surface MO to calculate the currents ibm, ibo and the ratio between the values ΔN1 and ΔN2 . For this purpose let us proceed from general consideration to the specific models of processes on the boundary MO. The most simple and real vacancy model is presented below. It allows to open a better physical sense of the relations (3).
The vacancy model of MO boundary
Vacancy model proceeds from the idea about the fact that, for forming the new cell M2O3 , three ions O2 -ok from the passivating layer must penetrate in the boundary mono layer of metal. This requires the presence of three vacancies Vm in it, which can arise only as a result of previous transition in the passivating layer of three ions of metal. These ions fill three vacancies Vom in the cation’s sublattice of oxide.The transition scheme
(Vom – vacancy in a metallicsublattice of oxide, Vm – vacancy in the boundary layer of the metal ΔM )
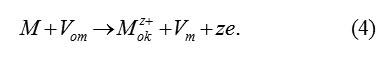
Transition (4) serves as the necessary condition for the subsequent penetration into the layer ΔM of oxygen ions
![]()
Where Vok– vacancy in the oxygen sub lattice in place passed into the metal O2-Ok ion.
The opposite reactions (4), (5)- appear basic in the vacancy model. It is obvious, that the surface MO serves as a drain of cationic vacancy Vom , brought by their diffusive flow jmv from solution. Simultaneously, it is the source of the vacancies Vok, which are discharged into the solution by their flow jov. Flows jmv and jov appear, of course, as a result of the motion of ions Mz+ok and O2–ok in the passivating layer by the mechanism of hopping migration in the strong field.
The transformation of vacancies and their flows occurs on the surface MO by the scheme.
In this transformation, the vacancies Vm are mediators, which connect processes (4) and (5). Their total quantity R is determined by the withdrawal of metal ions M and penetrationin metal of the oxygen ions O2–ok, i.e. the relations of the speeds (4) and (5).In the stationary mode, the value of R is invariable ( see below).
The speeds of formation of oxide and cation transfer through the boundaryMO.
Thus, processes On the boundary MO are characterized by the currents (speeds) ibm, Ibo of reactions (4), (5) and by the total current ibof the metal dissolution.It is measured, and is primary value, and its determination (3) can be called extra-model. The calculation of currents ibm, Ibo depends on model presentations. let us examine these values.
1) Transition current ibm of ions M in the passivating layer and their further transfer to the solution depends on the electric field E and the boundary concentration amNmv2 of vacancies Vom. Indeed, for transition into the passivating layer according to the scheme (4), ions M must cross the plane MO and move away from it on interatomic distance am of a cationic sublattice, having overcome potential jump Φm =amE. Transition barrier is reduced on ΔWm=αbzmamE, its probability is proportional to a quantity of vacant places, i.e., the concentration amNmv2 of vacancies Vm. As a whole the speed (4) is equal
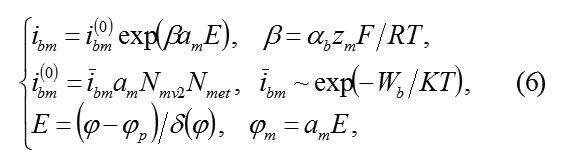
αb– transfer coefficient, Nmet – concentration of atoms in metal,ibm– speed constant of reaction.
2) The current ibo of ions M, which form oxide MOz/2 according to the reaction (5) , represents the speed of oxide formation on the Boundary MO.let us take it equal to (ibo – constant of speed, reverse current is neglected)
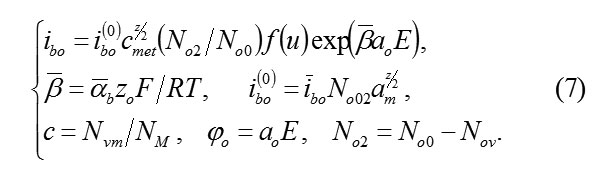
In the system (7), the dependence of current ibo on the field E, the concentration cmet , the vacancies Vm and the probabilistic factor f(u) are taken into account. the influence of field E is regulated by the value of transfer coefficient αb , and it is connected with the fact, that for the penetration into the metal through the plane MO, ion O2-ok must dislocate in the anion sublattice to the interatomic distance a0 , overcoming the potential jump φ0=a0E .In this case, the barrier of penetration is reduced to the value ΔW0=αbz0a0E. Influence on current ibo of concentration cmet considered with the fact, that without vacancies Vm, penetration can’t occur (see (5)).
The Probability factor f(u) in (7) takes into account the specific mechanism of formation of cells MOz/2. If the penetration of ions O2-ok and the structuring of cells occur in parallel, then it is not possible to exclude the deceleration necessary of regrouping of particles. The micro schemes of these processes can be different and random. Their possible influence on the speed of formation of oxide is described by probabilistic factorf(u) in (7). Its calculation is an autonomous task.
If the penetration of ions O2-ok is limited and it is weakly dependent on the subsequent structuring, then f(u)=1. Within the limit of the passivating layer it can consist of the partially structure systems complexes M2O3 , the speed of formation of which is determined by (7) at f(u)=1. Structuring depends, in particular, on the noncoincidence of interatomic distances and angular orientations in the lattices of metal and oxide.
Transfer coefficients αb and βb=1-αb and in (6) and (7) characterize asymmetry barrier W, which overcome by ions during their motion on the units of crystal lattice in the direct and opposite directions. Depending on α and β, how much an electric field changes barrier W. In the case of an isotropic gomogennmedium αb~ βb ~ ½ . However, in a thin passivating layer on the strange base layer, these values αb and βb are not proved. Furthermore, the current values αb , βb in the volume of the passivating layer, and its more disordered boundaries may differ, i.e they depend on coordinate system. Apparently, in this respect, the boundary MO is isolated, on which exactlyα= αb,β= βb. Then the jumps of the potential φm and φ0 overcome by ions M and O2–ok upon transition through the plane MO are equal respectively to φm=amE and φ0=a0E, Barriers(4) in the Boundary MO are reduced on ΔWm=αbzmφm and ΔW0=αbz0φ0 . All this are taken into account in (6) and (7).
Using (6), (7) it is possible to calculate stationary concentration of vacancies. Actually, the withdrawal of ions M and the penetration of ions O2–ok are strictly compensated (for example, for formation M2O3 , three ions M have to leave metal and three ions O2–ok will penetrate in it). for example, for forming , three ions must leave from the metal and three ions will penetrate in it.Therefore, speeds (6) and (7) are identical, i. e. 2/3ibm = ib0 and,consequently (excluding small reverse currents)
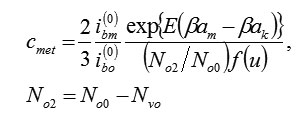
3) total current ib in the vacancy model is easy to relate with the values ibm and ib0 taking into account ,that the dissolution of the metal at the interface MO is faster oxide formation. Actually, according to the presented above, for the appearance of a cell M2O3 it is necessary for emergence of a cell, that: a) three ions of metal from a layerΔM passed to PL by the scheme (4), composing the current ibm; b) three ions O2-ok from a passivating layer enetrated into a layer ΔM according to the scheme (5); c) since, the cell M2O3 contains two ions Mz+ok, then two ions of metal in the layerΔM must be connected with three penetrating ions of oxygen O2-ok respectively, to form a complex M2O3 and to compose the current ib0 .
Thus, with the formation of each cell M2O3, layer ΔM loses not two, but five ions of metal, from which three prove to be excess.Their departure from the layer ΔM implements balance of space, compensating for the difference in specific volumes for each metal ion in the lattice of a metal oxide.Therefore in the passivating layer the intensive transfer of cations Mz+ok by the mechanism of hopping migration occurs. The surface MO is their intensive source, concentration Nmv2 of vacancies V0m in (6) is small, the relation of stationary values of currents m and ib0 equal 3/2.
The total current is equal to the sum ib = ibm + ib0 = 5/2ib0 = 5/2ibm or, accordingly (6),(3)
The current of the formation of oxide is equal ibo = 2/5ib. Every second on 1cm2 of the plane MO there is a number of cells M2O3 occurs, which at zm = 3 be equal
If the stoichiometry of oxide is different than the previous one, then it will change numerical coefficients [2]. In the general case for the cell MAOB we have
With the stated of thevacancy model boundaries MO, self processes at the interface with the opposite solution, considered in the following publications.
References
- Vetter K. Z. // Electrochemist. 1951. V. 55. P.274; 1954. V.58. P. 230; 1955. V. 59. P.67.
- Popov YU.A. // Electrochemist. 1985. V. 21. P. 1496; 1986. V. 22. P. 762; 1994. V. 30. P. 473.

This work is licensed under a Creative Commons Attribution 4.0 International License.