3-Hydroxy-3-n-Propyl-1-(4-Sulphonamidophenyl) Triazene: A New Reagent for Spectrophotometric Determination of Nickel (Ii)
Rehana Khanam1*, Saba Khan1 And Rekha Dashora2
1Department of Chemistry, Vidya Bhawan Rural Institute, Udaipur- 313001, India
2 Department of Chemistry, M.L.S.University, Udaipur-313001, India
DOI : http://dx.doi.org/10.13005/ojc/300260
Article Received on :
Article Accepted on :
Article Published : 22 May 2014
3-Hydroxy-3-n-propyl-1-(4-sulphonamidophenyl)triazene has been used for spectrophotometric determination of Nickel(II) at 400 nm, keeping the pH between 6.9 to 7.3. The Beer’s law is obeyed in the range 1x10-5 to6x 10-5 M. The molar absorptivity and Sandell’s sensitivity values are 6743 dm3 mol-1 cm-1 and 8.61 ng cm-2, respectively. The Nickel (II) has been determined successfully even in presence of upto 100 ppm of various interfering cations and anions. The reagent forms complex with iron at ratio of 1:2. The composition of complex was determined by Job’s method and Mole ratio method of Yoe and Jones. The value of log β found from two different methods were 9.57 and 9.53 respectively.
KEYWORDS:3-HYDROXY-3-n-PROPYL-1-(4-SULPHONAMIDOPHENYL); triazene has been used for spectrophotometric determination of Nickel(II)
Download this article as:| Copy the following to cite this article: Khanam R, Khan S, Dashora R. 3-Hydroxy-3-n-Propyl-1-(4-Sulphonamidophenyl) Triazene: A New Reagent for Spectrophotometric Determination of Nickel (Ii). Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Khanam R, Khan S, Dashora R. 3-Hydroxy-3-n-Propyl-1-(4-Sulphonamidophenyl) Triazene: A New Reagent for Spectrophotometric Determination of Nickel (Ii). Orient J Chem 2014;30(2) Available from: http://www.orientjchem.org/?p=3420 |
Introduction
A number of hydroxytriazenes1-7 have been used as spectrophotometric as well as metalochromic indicators. We report here spectrophotmetric determination of Nickel (II) using one such hydroxytriazene, 3-hydroxy-3-n-propyl-1-(4-sulphonamidophenyl) triazene.
Experimental
3 – Hydroxy – 3 -n- propyl-1- (4 -sulphonamidophenyl) triazene was synthesized by the reported method8 (Scheme:1).
Synthesis of 3-hydroxy-3-n-propyl-1-(4 -sulphonamidophenyl) triazene: 
Synthesis of n-propylhydroxylamine
In a one litre beaker 19.00g (0.30 mol) of nitropropane, 30 g of NH4Cl and 300 ml of distilled water were mixed, stirred mechanically and cooled to 0°C by surrounding the beaker with ice salt mixture. To the reaction mixture 30 g Zn dust was added in small lots in few minutes interval taking about an hour for complete addition. During this period, the temperature was maintained between 0° to 15°C by adding ice to the reaction mass occasionally. The reaction mixture was stirred mechanically for another 40 min keeping temperature between 0 to 15°C. The solution was filtered under suction and washed with 100 ml ice cold water. The filtrate was taken in a beaker and kept in freezer and used as such for coupling with diazotized product.
Diazotization of sulphanilamide
In a 500 ml beaker 0.2 mol of sulphanilamide was dissolved in warm mixture of 50 mL of concentrated HCl and 50 mL of water. After constant stirring the mixture was kept in a freezer to cool. In another beaker 13.9g of NaNO2 was dissolved in 40 mL of distilled water and kept it in the freezer. The beaker which contained sulphanilamide solution was put in an ice bath to maintain temperature between 0 to 5ºC. To this The NaNO2 solution was added drop by drop with continuous stirring. The diazotized product so obtained was directly used for coupling.
Coupling
The n-propylhydroxylamine prepared in step (a) was coupled with the diazotized product of (b) step at 0 to 5°C under mechanical stirring with occasional addition of sodium acetate solution for maintaining the pH close to 5 during coupling process. The compound 3-hydroxy-3-n-propyl-1-(4-sulphonamidophenyl) triazene was obtained as yellowish green powder after crystallization from ethanol. Melting points of all synthesized compounds were taken in open capillaries and are uncorrected. C H N analysis corroborated the purity of compound. The compound was subjected to IR spectral analysis and following bands were observed. IR (KBr) cm-1: 3290 (O-H str.), 3078 (C-H str. Ar), 2981 (C-H str., CH3), 1632 (N=N str.), 1419 (N-N str.).The spectra showed the compound to be in pure state. IR spectra (KBr) were recorded on FT IR RX1 Perkin Elmer Spectrometer. Physical and analytical data are given in Table 1 and the structure has been given in Figure 1.
Table 1: Elemental analysis of 3 – hydroxy – 3 -n-propyl – 1 – (4 -sulphonamidophenyl)
triazene (HPSPT)
|
Molecular Formula of HPSPT |
Molecular Weight |
M.P. ºC |
Elemental Analysis |
|||
|
C% |
N% |
H% |
||||
|
C9H14N4O3S |
258.30 |
180 |
Th. |
41.85 |
21.69 |
5.46 |
|
Exp. |
41.65 |
21.53 |
5.01 |
|||
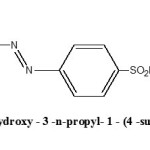 |
Figure 1 Click here to View Figure |
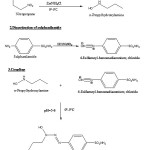 |
Scheme 1 Click here to View Scheme |
SPECTROPHOTOMETRIC DETERMINATION OF NICKEL (II) WITH HPSPT
Stock Solution
A 1.0x 10-2 M stock solution of Nickel chloride hexa hydrate (BDH) was prepared in distilled water. Few drops of (1 M) concentrated hydrochloric acid were added to prevent hydrolysis. The solution was standardized with EDTA solution at pH 10-11 using murexide9 as an indicator. A 1×10-2 M solution of the reagent 3-hydroxy-3-n-propyl-1-(4-sulphonamidophenyl) triazene was prepared in alcohol. Tris-buffer (1%, w/v) was prepared. A UV/VIS. Systronic 106 Spectrophotometer and a pH Scan 2 tester were used.
Method
Spectrum of 3-hydroxy-3-n-propyl-1-(4-sulphonamidophenyl) triazene was measured in the wavelength region 360 – 460nm against solvent blank. Nickel and 3-hydroxy-3-n-propyl-1-(4- sulphonamidophenyl) triazene solutions were taken in 1:5 ratio and the spectrum of Nickel complex was recorded against reagent blank in the range 360-460 nm. The working wavelength was found to be 400 nm. A set of solutions containing Ni(II) and 3-hydroxy-3-n-propyl-1-(4-sulphonamidophenyl) triazene reagent in ratio 1:5 was prepared and ph was varied between 5 to 8. The pH range of constant maximum absorbance was found to be between 6.9 to 7.3. Composition of the complex was determined by Job’s method and moles ratio method of Yoe and Jones. The study revealed that composition of Nickel (II) complex is 1:2 (M:R). Absorbance of set of six solutions containing Ni(II) to 3-hydroxy-3-n-propyl-1-(4-sulphonamidophenyl) triazene in ratio 1:5 was measured at corresponding working wavelength against reagent blank. Beer’s law was obeyed in concentration range 1×10-5 to 6×10-5 M. Interference of 23 cations and anions in the determination of nickel was studied. To the set of solutions containing nickel to reagent 1:5 ratio, 10 ppm of different foreign ions were added at optimum conditions. Absorbance was measured against reagent blank. Those ions, which did not interfere at 10 ppm level their interference was again studied at 50 ppm level. In case no or little change in absorbance was seen as compared to the absorbance without any foreign ion, then for those ions interference was studied again at 100 ppm level. However tolerance of still higher concentration was not studied.
Results and Discussion
Nickel (II) forms 1:2 complex with 3-hydroxy-3-n-propyl-1-(4-sulphonamidophenyl) triazene.
Stability constants
Harvey and Manning’s method10 and Purohit’s method11 have been used to determine the stability constants. Validity of the methods can be confirmed from the value of log β obtained from both the methods. Values of log β obtained by Harvey and Manning method and Purohit’s method were 9.57 and 9.53 respectively. The log β values agree quite well. Further the precision studies were carried out by measuring the absorbance of 10 sets of solution containing 5.86 ppm of Nickel (II), and title reagent in 1:5 ratio, under optimum conditions. The absorbance was measured against reagent blank at working wavelength (400 nm). Nickel was successfully determined at 5.86 ppm level with good precision. The value of ΔG obtained for the Harvey and Manning method and Purohit’s method were – 13.13 and –13.14(kcal/mole) at 27°C respectively.
Interference Studies
Interference of several cations and anions in the determination of nickel was studied at 10, 50 and 100 ppm level. Interference was studied using following 23 cations and anions viz. Na+, K+, NH4+,Ba2+, Mn2+ Co2+, Pb2+, Cu 2+, Zn 2+, Cd2+, Mg2+, F–,Cl–, Br –, I–, NO2–, NO3–, SO4-2, WO4-2, CO32-, S2O32- ,C2O42- ,CH3COO–. It was seen that at 100 ppm levelthe ions which still did not interfere are, Na+, K+,NH4+, Ba2+, Mg2+, Cl– , Br –, I–. F–, SO4-2, WO4-2, NO2–, NO3–, CO32-, CH3COO–. However tolerance of higher concentration was not studied. Thus it can be seen that Nickel (II) can be determined even in presence of number of interfering species present at 100 ppm level.
Sandell’s Sensitivity
The molar absorptivity of complex was calculated from the Beer’s law graph and was found to be ε=6743 dm3 mol-1 cm-1.The value thus obtained was used for determining Sandell’s spectrophotometric sensitivity of the complex which was 8.61 ng cm-2. This value is very low as compared to the following most commonly used reagents viz., 2-Acetylthiophenethiocyanate (48.92 ng cm-2)12, N,N’-Bis-(2-hydroxy-4-n-butoxyaceto-phenylidine) ethylenediamine (41.65 ng cm-2)13,1-(2,3,5Triazolylazo)-2-naphthol(17.26 ng cm-2)14, Sulphosalicylic acid(87.73 ng cm-2)15, Ethylenediaminetetraaceticacid (3952.19 ng cm-2)16, Dimethylglyoxime(14.67 ng cm-2)17, 4-Methyl-2,3-pentanedionedioxime(19.31 ng cm-2)18.
Conclusions
Thus the present studies have introduced 3-hydroxy-3-n-propyl-1-(4-sulphonamidophenyl) triazene as new reagent for nickel (II) determination spectrophotometrically which is quite comparable to most of the spectrophotometric reagents being used in the nickel (II) determination. Its easy synthesis, higher yields and economic method for preparation further enhance its applicability as spectrophotometric reagent for nickel(II) determination.Thus from the above studies it can be concluded that 3-hydroxy-3-n-propyl-1-(4-sulphonamidophenyl) triazene can be used successfully for spectrophotometric determinationof Nickel(II). The tentative structure of 1:2 complex of Ni (II) with HPSPT has been given in Figure2.
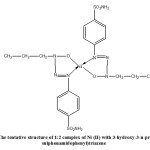 |
Figure 2 Click here to View Figure |
References
- Purohit D N, Talanta, 14 (1967) 353.
- Chakrovorty D & Majumdar A K, J .Ind.Chem. Soc., 54(1977) 258.
- Dutta R L and Sharma R, J. Sci. Ind. Res., 40 (1981) 715.
- Purohit D N, Nizzamuddin & Golwalker A M, Revs. Anal. Chem. (Israel), 8 (1985) 76.
- Purohit D N, Tyagi M P, Bhatnagar R & Bishnoi I R, Revs. Anal. Chem. (Israel), 11 (1992) 269.
- Gorgi D K, Chauhan R S , Goswami A K & Purohit D N , Rev. Anal. Chem. (Israel), 17 (1998) 223.
- Kumar S, Goswami A K & Purohit D N, Rev. Anal. Chem. (Israel), 22( 2003) 73.
- Mitsuhashi T & Simamura O, J.Chem.Soc.(London)B( 1970), 705.
- Vogel A I, A textbook of quantitative Inorganic analysis, Third Edition, ELBS.
- Harvey A E & Manning D L, J.Am. Chem.Soc., 72(1950) 4488; 74,( 1952) 4744.
- Ressalan S , Chauhan R S , Goswami A K & Purohit D N , Asian J. Chem., 11, 1999, 123.
- Lokhande R S, Chaudhary A B, Nirupa S, Asian J.Chem., 14 (2002) 153.
- Parikh K S, Acta Cienc.Indica, 27(2001) 49.
- Jiang Huahong, ye Qiaoyun, Huaxue Shic, , 42(2001) 579.
- Bhat N Gopalkrishna, Narayana B, Nazareth R A, SreeKumar N V , Nambiar C H R, J.Indian.Chem.Soc., 80(2003) 938.
- Carpani Irene, Scavetta Erika & Tonelli Domenica, Annali di Chimica(Rome, Italy), 94(2004)365.
- Wu Shengjum, Wu Taikai, Wan Jiebao, Fujian Fenxi Ceshi, 12(2003) 1830.
- Garole D J, Sawant A D, J.Sci.Ind.Res., 64(2005) 581.

This work is licensed under a Creative Commons Attribution 4.0 International License.









