The Influence of Tyrozine on Energetic Property in Graphene Oxide: A DFT Studies.
Roya Ahmadi and Reza Soleymani *
Department of Chemistry, Shahre-Rey Branch, Islamic Azad University, Tehran, Iran
DOI : http://dx.doi.org/10.13005/ojc/300107
Article Received on : December 15, 2013
Article Accepted on : January 31, 2014
Article Published : 30 Mar 2014
Using the Computational methods, the interaction effect of Tyrosine Amino acid on Graphene was investigated. For this purpose, the Density Functional Theory )DFT (in the ground state of 6-31G was used, and the interaction effects of Tyrosine on Graphene was investigated through attachment to three different base positions. Different parameters such as energy levels, the amount of Chemical Shift in different atoms, the amount of HOMO/LUMO values and related parameters like Electrophilicity scale, chemical hardness, Chemical potential, and the maximum amount of electronic charge transferred. The Graphene oxide has the capability to act as adrug nano carrier and also as a mixture with special electrical properties. The results of this investigation also show that the attachment of Tyrosine Amino acid, as an organic compound, to the chemical structure of Graphene can change these capabilities to a great extent and also increase the role that this mixture already plays in medical, Pharmaceutical, and electronic industries.
KEYWORDS:DFT; Tyrosine; HOMO/LUMO; Electrophilicity
Download this article as:| Copy the following to cite this article: Ahmadi R and Soleymani R. The Influence of Tyrozine on Energetic Property in Graphene Oxide: A DFT Studies. Orient J Chem 2014;30(1). |
| Copy the following to cite this URL: Ahmadi R and Soleymani R. The Influence of Tyrozine on Energetic Property in Graphene Oxide: A DFT Studies. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2583 |
Introduction
Nanostructures can be categorized into following forms according to their structures: diamonds with SP3 hybridization, Graphites with SP2 hybridization, Hexagonal diamonds with SP3 hybridization, fullerenes with SP2 hybridization, Nanoparticles, Graphenes, single-layer and multi-layer nanotubes, Crystal Nanostructures. All these forms of nanostructures produce unique Pharmaceutical and electronic properties. Graphenes have a two-dimensional structure of a single layer of carbon chicken wire [1-5]. The production of Graphene from Graphete acid began in the 19th century and the most common methods for this production were Brodie, Staudenmaier, and Hummers. But Graphene oxide is one of the derivatives of Graphene which is produced mainly by Brodie, Staudenmaier, and Hummers methods. All these methods oxidize the Graphete, using strong acids and oxidizers. The oxidation level of this process is dependent upon the kind of method, the type of the Graphete, and also the circumstances of the chemical interaction. The Graphene oxide can be used for a number of purposes such as saving energy, acting as a chemical sensor, and also acting asadrug nano carrier [5-9]. Tyrosine is a group of amino acids which are synthesized in the body from phenylalanine. It is found in in the structure of the most of the body’s proteins. Tyrosine is also found in the structure of most of the neurotransmitters such as Dopamine, L- Dopamine, Epinephrine, and Norepinephrine. Because of the role that tyrosine plays in the structure of neurotransmitters, it can probably have an effect in the emergence and improvement of diseases like Parkinson’s disease, depression, and many other kinds of mental disorders. The findings shoe that L-Tyrosine can be beneficial for those who suffer from depression and can be used for the treatment of diseases of this kind. The early researches show that L-Tyrosine can also be beneficial to those who suffer from brain diseases such as Alzheimer’s disease. It has been found that Because of the role that this amino acid plays in the structure of Epinephrine and Norepinephrine, it can help reduce the environmental, physical, and mental stress. In the skin cells, Tyrosine converts into a substance called Melanin. This substance can protect the skin from ultraviolet rays. Tyrosine is also used in the structure of thyroid hormones which play an important role in body’s metabolism. The persons who excrete a lot of proteins due to Kidney diseases, are open to deficit of amino acids, including Tyrosine amino acid [10]. Moreover, some persons genetically suffer from a disorder called Phenylketonuria. The Electrophilicity parameter was first defined and investigated by Parr et al. the Electrophilicity parameter can be used in the investigation of the most of the systems and also in the description of the chemical reactions in different organic ways. Besides, in some of the chemical reactions, this factor has been regarded as an effective factor in the amount of outcome in Diels–Alder reactions. The Electrophilicity parameter is caused by the electronic structure of the substance and is independent from the effects of molecular nucleus. In a series of studies, Domingo et al investigated the relation among the electronic effects of substitutions, electrophilicity parameter, and Hammett equation in a mixture of ethylene. The Electrophilicity values, the maximum amount of electronic charge transferred, chemical hardness, and also Chemical potential can be calculated using the relations 1 to 4. In these relations, the (I) represents “ionization potential” and (A) stands for “electron affinity” [13-21].
Formula 1,2,3,4,5,6
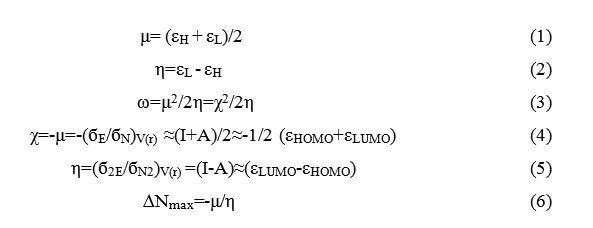
Computational details
All these calculations are done under the assumption of standard state of gas phase, pressure of 1 atmosphere, and temperature of 25 degrees centigrade. The calculations are performed, using a Pentium 4 PC with a Windows 7 OS and a Core i7 processor. After the initial optimization of the mixtures, the Gaussian 09 software is used for final optimization. For the calculation of HOMO/LUMO values, the Gauss sum software is used [18, 21].
Result and discussion:
The related structures are named in the following way: GO: Graphene oxide without any attachment to structure of Tyrosine Amino acid GO- B1: Graphene oxide when attached to the first base position in the structure of Tyrosine Amino acid GO- B2: Graphene oxide when attached to the second base position in the structure of Tyrosine Amino acid GO- B3: Graphene oxide when attached to the third base position in the structure of Tyrosine Amino acid. In GO-B1, the attachment of Tyrosine structure to Graphene oxide is done through the attachment of O45 in Graphene oxide structure to N70 in Tyrosine structure. In GO-B2, the attachment of Tyrosine structure to Graphene oxide is done through the attachment of C43 in Graphene oxide structure to N69 in Tyrosine structure. In GO-B3, the attachment of Tyrosine structure to Graphene oxide is done through the attachment of O67 in Graphene oxide structure to N70 in Tyrosine structure. One of the parameters that can be calculated by Computational chemistry is the value of HOMO and LUMO and their related parameters such aschemical hardness, Chemical potential, Electrophilicity, the most amount of surroun dedelectric charge, and the energy gap. The results of the study are tabulated in the table NO 1.
Table 1. Values of energies of the frontier molecular orbitals (εHOMO and εLUMO, eV), electronic chemical potential, μ (eV), chemical hardness, h (eV), electrophilicity, ω (eV) and maximum amount of electronic charge transfer for all of Geraphene Oxide structures calculated at the B3LYP/6-31G level of theory.
|
Method |
B3LYP/6-31G |
||||
|
Structure |
GO-B1 |
GO-B2 |
GO-B3 |
GO |
TR |
|
HOMO |
-0.19611 |
-0.19327 |
-0.19007 |
-0.19811 |
-0.21308 |
|
LUMO |
-0.17867 |
-0.17562 |
-0.17349 |
-0.18077 |
-0.01807 |
|
Chemical Potential |
-0.18739 |
-0.18444 |
-0.18178 |
-0.18944 |
-0.11557 |
|
Chemical Hardness |
0.01744 |
0.01765 |
0.01658 |
0.01734 |
0.19501 |
|
Electrophilicity |
1.00673 |
0.96373 |
0.99650 |
1.03481 |
0.03424 |
|
ΔNMax |
10.7448 |
10.4501 |
10.9638 |
10.9250 |
0.59266 |
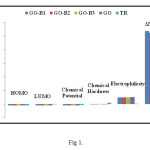 |
Fig. 1. Displays the trend of the HOMO/LUMO changes in the various charts. Click here to View Figure |
As the figure No 1 shows, the attachment of Tyrosine changes the trend of values related to HOMO and LUMO. For comparing the energetic level of structures, after the optimization of the structures by Density Functional Theory at a theory level of B3LYP, different structural forms are compare with one another. The results of this comparison which are tabulated in table No 2 show that the attachment of Tyrosine structure to the structure of Graphene oxide changes the values of energy.
Table 2. The values of different position energies for Geraphene Oxide calculated at the B3LYP/6-31G level of theory.
|
Method |
B3LYP/6-31G |
||||
|
Structure |
GO-B1 |
GO-B2 |
GO-B3 |
GO |
TR |
|
Total energy (Hartree) |
-3325.4514 |
-3250.3262 |
-3325.4580 |
-2697.0456 |
-629.6277 |
|
ZPVE (Kcal/mol) |
405.55973 |
403.52363 |
405.23424 |
296.28579 |
121.51554 |
|
Rotational constant (GHz) |
0.04677 |
0.05419 |
0.04521 |
0.06565 |
2.34377 |
|
0.02055 |
0.01976 |
0.03207 |
0.04739 |
0.33704 |
|
|
0.01634 |
0.01589 |
0.02276 |
0.03083 |
0.31371 |
|
|
Entropy (Cal/Mol-Kelvin) |
|||||
|
Total |
308.700 |
307.304 |
305.375 |
246.615 |
111.970 |
|
Translational |
46.304 |
46.251 |
46.304 |
45.652 |
41.488 |
|
Rotational |
41.145 |
41.065 |
40.406 |
39.346 |
31.540 |
|
Vibrational |
221.251 |
219.988 |
218.664 |
161.617 |
38.943 |
|
|
|||||
|
Heat capacity (Cal/Mol-Kelvin) |
212.111 |
208.502 |
212.460 |
165.635 |
47.226 |
|
Dipole moment(D) |
4.6276 |
6.7374 |
4.6509 |
4.5859 |
1.8667 |
 |
Fig. 2. Partial DOS diagram containing HOMO (left) and LUMO (right) plot of Graphene Oxide derivatives at B3LYP method. Click here to View Figure |
 |
Fig. 3. Displays the trend of the energetic property changes in the various charts. Click here to View Figure |
Conclusion
Computational Quantum Mechanics at the theory level of B3LYP/6-31G on the structure of Graphene oxide was done separately and only when the structure of Tyrosine was attached to it and the results of this computation can be classified as follows: The investigation of all the parameters show that the attachment of Tyrosine structure to Graphene oxide structure will influence the energy levels and dipole moment changes and these changes are able to be investigated in the electrical and chemical parameters of Graphene oxide structure. The attachment of Tyrosine structure is a reason for the changes in the energy levels and also HOMO and LUMO values. The investigation of energy levels and HOMO/LUMO values show that the attachment of Tyrosine structure to Graphene oxide structure form different positions of base will yield different results for chemical hardness, chemical potential, Electrophelicity, and ∆Nmax, in accordance to the attachment position; and the most appropriate position is the most symmetrical one. We expect that the changes in chemical parameters change the physical, electronic, and even pharmaceuticalproperties of Graphene oxide and Tyrosine, and these changes are able to be investigated by the specialists of these fields.
Acknowledgements
The authors are indebted to Dr Sargordan-Fard Arani for their interest in this work and many helpful discussions. Moreover, this work was supported by Islamic Azad University Shahre-Rey branch.
References
- K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dobonos, Science, 306, (2004), 666.
- K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Nature, 438, (2005), 1970.
- M. Terrones, A. Botello-Mendez, J. Campos-Delgado, F. Lopez-Urias, Nano Today, 5, (2010), 351.
- B.C. Brodie, Ann. Chim. Phys, 59, (1860), 466.
- W.S. Hummers, R.E. Offeman, J. Am. Chem. Soc, 80, (1958), 1339.
- S. Stankovich, D.A. Dikin, G.H.B. Dommett, Nature, 442, (2006), 286.
- Z. Gomez, R.T. Weitz, A.M. Bittner, M. Scolari, A. Mews, Nano Letters, 7, (2007), 3499.
- W.W. Cai, R.D. Piner, F.J. Stedemann, S. Park, R.S. Ruoff, Journal of Physical Chemistry, 321, (2008), 1815.
- W. Gao, L.B. Alemany, L. Ci, P.M. Ajayan, Nature Chemistry, 1, (2009).
- G.C. Barrette, D.T. Elmore, Amino Acids and Peptides, Cambridge University Press, (1998).
- Reza Soleymani, Reihaneh Dehghanian Dijvejin, Asiyeh Fallahi Gozal Abad Hesar, Oriental Journal of Chemistry, 28 (3), (2012), 1107.
- Reza Soleymani, Halimeh Rajabzadeh, Asian Journal of Chemistry, 24 (10), (2012), 4614.
- Goldasteh Zarei, Reza Soleymani, Reihaneh Dehghanian Dijvejin, Oriental Journal of Chemistry, 28 (3), (2012), 1229.
- Roya Ahmadi, Reza Soleymani, Tahereh Yousofzad, Oriental Journal of Chemistry, 28 (2), (2012), 773.
- Reza Soleymani, Reihaneh Dehghanian Dijvejin, Oriental Journal of Chemistry , 28 (3), (2012), 1291.
- Ehsan Fereyduni, Mahdi Kamaee, Reza Soleymani, Roya Ahmadi, Journal of Theoretical and Computational Chemistry, 11(6), ( 2012). 1331.
- Reza Soleymani, Farzad Torkashvand, Sahar Farsi-Madan, Mohammad Bayat, Oriental Journal of Chemistry, 28 (2), (2012), 733.
- Reza Soleymani, Khalil Ghesmat-Konandeh, Reihaneh Dehghanian Dijvejin, Oriental Journal of Chemistry, 28 (3), (2012), 1331.
- Reza Soleymani, Sahar Farsi-Madan, Khalil Ghesmat-Konandeh, Oriental Journal of Chemistry, 28 (2), (2012), 703.
- Reza Soleymani, Yasin Mohammad Salehi, Tahereh Yousofzad, Maryam Karimi-Cheshmeh Ali, Oriental Journal of Chemistry, 28 (2), (2012), 627.
- M.J. Frisch, et al., Gaussian 98. Gaussian, Inc., Pittsburgh, PA, (1998).

This work is licensed under a Creative Commons Attribution 4.0 International License.









