Synthesis, Characterization, and Crystal Structure Determination of a New Indium (III) Complex: [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO)
Sadif A. Shirvan*, Fereydoon Khazali, Sara Haydari Dezfuli
Department of Chemistry, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran.
DOI : http://dx.doi.org/10.13005/ojc/300154
Article Received on :
Article Accepted on :
Article Published : 06 Feb 2014
[In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO) complex (5,5’-DiMeBiPy is 5,5’-Dimethyl-2,2’-bipyridine) was prepared from reaction of InCl3.4H2O with 5,5’-DiMeBiPy in 1.1 molar ratio in DMSO. This compound has been characterized by IR, 1H NMR, UV-Vis spectroscopy as well as X-ray crystallography. This compound crystallizes in the space group Pī of the triclinic system. The unit cell dimensions is: a = 8.6695(8) Å, b = 12.8456(14) Å, c = 13.0877(12) Å, a = 78.258(8)º, β = 85.270(8)º, g = 74.932(8)º. According to X-ray structure determination, there are one molecules of the complex and two DMSO solvent molecules in the asymmetric unit. In this complex the geometry at the indium (III) center is octahedral, formed by two nitrogen atom of 5,5’-DiMeBiPy ligand, three chloride ions and one DMSO molecule in trans position to chloride ion.
KEYWORDS:Indium (III); 5,5’-Dimethyl-2;2’-bipyridine; DMSO; Crystal structure.
Download this article as:| Copy the following to cite this article: Shirvan A. S, Khazali F, Dezfuli S. H, .Synthesis, Characterization, and Crystal Structure Determination of a New Indium (III) Complex: [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO). Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Shirvan A. S, Khazali F, Dezfuli S. H, .Synthesis, Characterization, and Crystal Structure Determination of a New Indium (III) Complex: [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO).Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2037 |
Introduction
Metal complexes containing 2,2’-bipyridine and phenanthroline ligands have gained importance due of their versatile roles as molecular scaffolding for supramolecular assemblies, and due to their applications in catalysis, electrochemistry biochemistry and ring-opening metathesis polymerization and biochemistry [1-8].
On the other hand, the chemistry of indium compounds is of interest owing to their important application in catalysts, electronics and optics [9-12]. Several In(III) complexes, which have the formula [In(N-N)Cl3(Solv)], (Solv = DMSO, DMF, H2O, MeOH and EtOH), are known, such as [In(BiPy)Cl3(H2O)], [In(BiPy)Cl3(EtOH)], and [In(BiPy)Cl3(H2O)].H2O, [13], [In(Phen)Cl3(DMSO)], [14], [In(4,4′-DiMeBiPy)Cl3(DMSO)], [15], [In(Phen)Cl3(H2O)], [In(Phen)Cl3(EtOH)].EtOH, [16], [In(5,5′-DiMeBiPy)Cl3(MeOH)], [17] and [In(4,4′-DiTertBuBiPy)Cl3(MeOH)].CH3OH, [18] (where BiPy is 2,2′-bipyridine, Phen is 1,10-phenanthroline, 4,4′-DiMeBiPy is 4,4′-dimethyl-2,2′-bipyridine, 5,5′-DiMeBiPy is 5,5′-dimethyl-2,2′-bipyridine and 4,4′-DiTertBuBiPy is 4,4′-ditertiarybuthyl-2,2′-bipyridine).
We recently reported the coordination chemistry of In(III) with some substituted bipyridine ligands such as [In(4,4′-DiMeBiPy)Cl3(MeOH)].CH3OH, [19], [In(5,5′-DiMeBiPy)Cl3(DMF)],H2O, [20] and [In(6-MeBiPy)Cl3(DMSO)], [21] (where 6-MeBiPy is 6-methyl-2,2′-bipyridine). Herein the synthesis, characterization and crystal structure of a new indium(III) complex containing 5,5’-Dimethyl-2,2’-bipyridine ligand by the formula of [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO) have been reported.
Experimental Section
Materials and instruments
All chemicals were purchased from Merck and Aldrich. Infrared spectra (4000-250 cm-1) of solid samples were taken as 1% dispersion in KBr pellets using a Shimadzu-470 spectrometer. 1H- and 13C-NMR spectra were recorded on a Bruker AC-300 MHz spectrometer operating in the quarter mode.
Synthesis of [In(5,5′-dmbipy)Cl3(DMSO)].2DMSO
5,5′-Dimethyl-2,2′-bipyridine (0.20g, 1.10 mmol) in methanol (5 ml) was added to a solution of InCl3.4H2O (0.32g, 1.10 mmol) in methanol (5 ml) and the resulting colorless solution was stirred for 30 min at 40°C. The suitable crystals for the X-ray diffraction experiment were obtained by methanol diffusion to a colorless solution in DMSO. Suitable crystals were isolated after one week (yield 0.54 g, 76.7%). IR (CsI, cm-1): 3105m, 2993m, 2918, 1588m, 1514m, 1478s, 1396m, 1330s, 1313m, 1256m, 1141m, 1050s, 986m, 944s, 868m, 840s, 715m, 645w, 525m, 425m, 394m, 343m, 310m, 275m. 1H NMR (DMSO-d6, ppm): 2.63 (s, 3 H), 8.40 (d, 1 H), 8.83 (d, 1 H) and 9.28 (s, 1 H). 13C NMR (DMSO-d6, ppm): 19.3 (s), 126.1 (s), 141.3 (s), 144.9 (s), 147.6 (s) and 152.6 (s). UV-Vis: λmax (DMSO, nm), 295. Anal. Calcd for C18H30Cl3InN2O3S3 (%): C, 33.79; H, 4.69; N, 4.38. Found: C, 33.50; H, 4.65; N, 4.34.
Crystal structure determination and refinement
The X-ray diffraction measurements were made on a Bruker SMART 1000 CCD area detector diffractometer at 298 K (Mo-Kα radiation, graphite monochromator, λ = 0.71073 Å). The structure was solved by SHELX-97 and
SHELXTL and absorption correction was done using the SADABS and APEX2 programs [22, 23]. Data collection, cell refinement and data reduction were done by APEX2, SAINT, SHELXTL, PLATON and MERCURY [22-25].
Results and discussion
Synthesis.
Compound [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO) was obtained from reaction of one equivalent of InCl3.4H2O with one equivalent of 5,5’-Dimethyl-2,2’-bipyridine in dimethylsulfoxide at room temperature, in 63% yield, Eq. (1):
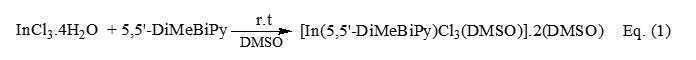
The suitable crystal for X-ray diffraction experiment was obtained by slow evaporation of DMSO in room temperature after three weeks. Synthesized complex was thoroughly characterized by elemental analysis, IR and 1H-NMR spectroscopy. Elemental analysis data (C, H, N) support the general composition of the complex and the structures have been established by single-crystal X-ray diffraction study.
NMR, IR and Uv-vis investigation.
Compound [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO) is stable in air and can be used directly for routine analyses. The vibrational band present at 3105 cm-1 is assigned to ν (C-Hcycle)and two vibrational bands present at 2993 and 2918 cm-1 are assigned to ν(C-HMe). The several bands in the range 1588-1313 cm-1 are assigned to ν(C=N) and ν(C=C) vibrations. Medium to strong vibration bands in the range 986-525 cm-1 are assigned to deformation vibrations δ(C=C=N) and δ(C=C=C) in the pyridine rings [26-30]. In addition, two strong signals at 1050 and 944 cm-1 has been assigned to ν(S=O) for free and coordinated DMSO, respectively [31-33]. Far infrared
![Fig 1. The labeled diagram of [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO). Thermal ellipsoids are at 50% probability level.](http://www.orientjchem.org/wp-content/uploads/2014/02/Vol30_No1_Synth_Sad_Fig1-150x150.jpg) |
Fig 1. The labeled diagram of [In(5,5’ DiMeBiPy)Cl3(DMSO)].2(DMSO). Thermal ellipsoids are at 50% probability level. |
spectra for title complex were recorded between 425 and 264 cm-1. In-O stretching vibration is seen at 425 cm-1. The In-N stretching vibration is seen at 394 and 343 cm-1 and In-Cl stretching vibration is seen at 310 and 275 cm-1 [31-33].
Table 1. Crystallographic and structure refinements data of [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO).
| Complex | |
| Formula | C18H30Cl3InN2O3S3 |
| Formula weight | 639.82 |
| Temperature /K | 298(2) |
| Wavelength λ /Å | 0.71073 |
| Crystal system | Triclinic |
| Space Group | Pī |
| Crystal size /mm3 | 0.50×0.40×0.35 |
| a /Å | 8.6695(8) |
| b /Å | 12.8456(14) |
| c /Å | 13.0877(12) |
| α /° | 78.258(8) |
| β /° | 85.270(8) |
| γ /° | 74.932(8) |
| Volume / Å3 | 1377.3(2) |
| Z | 2 |
| Density (calc.) /g cm-1 | 1.543 |
| θ ranges for data collection | 2.43-26.00 |
| F(000) | 648 |
| Absorption coefficient | 1.397 |
| Index ranges | -10 ≤ h ≤ 10 |
| -15 ≤ k ≤ 15 | |
| -16 ≤ l ≤ 15 | |
| Data collected | 10935 |
| Unique data (Rint) | 5378, (0.0963) |
| Parameters, restrains | 272, 0 |
| Final R1, wR2a (Obs. data) | 0.0581, 0.1513 |
| Final R1, wR2a (All data) | 0.0663, 0.1566 |
| Goodness of fit on F2 (S) | 1.057 |
| Largest diff peak and hole /e Å3 | 1.479, -1.548 |
The UV-Vis spectrum of the DMSO solution of 1 have band at 295 nm which can be assigned to the π→*π transition [29]. NMR spectroscopy studies were conducted to characterize the chemical structure of title complex. A comparison between 1H and 13CNMR spectra of title complex and 5,5′-dmbpy ligand, clearly indicated the coordination of ligand to In(III). It can be deduced from NMR data that in the solution, the 5,5′-dmbpy ligand have a symmetrical environment. As expected, the 1H NMR spectrum exhibited a singlet at 2.63 ppm for methyl, a doublet at 8.40 ppm, a doublet at 8.83 ppm and a singlet at 9.28 for the aromatic rings. The 13C NMR spectrum showed a singlet at 19.3 ppm, for CH3 groups and five singlets at 126.1 to 152.6 ppm for the aromatic rings.
Description of the molecular structure of [In(5,5’-DiMeBiPy)Cl3(DMSO)]. 2(DMSO).
Table 2. Selected bond distances (Å) and bond angles (°) of [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO).
| Bond distances | |||
| In1-N1 | 2.293(4) | In1-Cl2 | 2.4354(14) |
| In1-N2 | 2.285(4) | In1-Cl3 | 2.4600(15) |
| In1-Cl1 | 2.4190(16) | In1-O1 | 2.250(4) |
| Bond angles | |||
| Cl1-In1-Cl2 | 99.61(6) | N1-In1-Cl1 | 164.85(12) |
| Cl1-In1-Cl3 | 98.33(6) | N1-In1-Cl2 | 93.52(11) |
| Cl2-In1-Cl3 | 97.07(6) | N1-In1-Cl3 | 87.43(11) |
| O1-In1-Cl1 | 90.23(11) | N1-In1-N2 | 71.90(15) |
| O1-In1-Cl2 | 87.81(11) | N2-In1-Cl1 | 93.85(12) |
| O1-In1-Cl3 | 169.26(10) | N2-In1-Cl2 | 162.74(11) |
| O1-In1-N1 | 82.71(14) | N2-In1-Cl3 | 91.58(11) |
| O1-In1-N2 | 81.33(14) |
The colorless prismatic crystals of [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO) were grown by slow evaporation of DMSO solution during three weeks. Table 1shows details of collected data and refinement of the X–ray crystal structure determination for this compound. Selected bond lengths and bond angles are presented in Table 2. The crystal structure of this complex consists of 3 chloride ion, one chelating 5,5’-Dimethyl-2,2’-bipyridine and one DMSO. Two DMSO molecules are in the unit cell as solvent. ORTEP view with numbering scheme and packing diagram is shown in Figure 1. The complex have distorted octahedral geometry. In this complex indium has pseudo-octahedral coordination with fac-arrangement of
chlorine atoms. In-Cl bond lengths differ strongly around indium center. The bond lengths In1-Cl3 (chlorine atom is trans to oxygen) is longer than distances In1-Cl1 and In-Cl2 [C11 and Cl2 atoms are trans to nitrogen atoms].
![Fig 2. Crystal packing diagram of [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO) showing the C-H…Cl and C-H…O hydrogen bonds and π…π stacking interactions between adjacent molecules.](http://www.orientjchem.org/wp-content/uploads/2014/02/Vol30_No1_Synth_Sad_Fig2-150x150.jpg) |
Fig 2. Crystal packing diagram of [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO)showing the C-H…Cl and C-H…O hydrogen bonds and π…π stacking interactions between adjacent molecules. |
As it is shown in Figure 2, the 5,5’-Dimethyl-2,2’-bipyridine ligands form π bonding stacks in which each bipy ligand lies between two bipy ligands of adjacent indium complexes. The centroid to centroid distance between adjacent aromatic rings are 3.605 and 3.937 Å. Weak C-H…Cl and C-H…O hydrogen bonds are linked adjacent molecules in another direction to generate a 3D packing,
Table 3. Hydrogen bond geometry of [In(5,5’-DiMeBiPy)Cl3(DMSO)].2(DMSO) in crystal packing (Å, °).
| D-H…A | D-H | H…A | D…A | D-H…A | Symmetry code |
| C1-H1···Cl2 | 0.93 | 2.780 | 3.437(5) | 129 | – |
| C12-H12···Cl1 | 1.0(2) | 2.780 | 3.3438(7) | 128 | – |
| C14-H14C···Cl2 | 1.0(2) | 2.750 | 3.565(10) | 143 | 2-x,1-y,1-z |
| C13-H13B···O2 | 0.96(1) | 2.625 | 3.439(8) | 142 | x,1+y,z |
Acknowledgments
We would like to thank the Islamic Azad University, Omidieh Branch for financial support.
References:
- Chow C. S., Bogdan F. M., Chem. Rev., 97, 1489 (1997).
- Sammes P. G., Yahioglu G. Chem. Soc. Rev., 23, 327 (1994).
- Balzani V., Juris A., Venturi M., Campagna S., Serroni S., Chem. Rev., 96, 759 (1996).
- Calderazzo F., Pampaloni G., Passarelli V., Inorg. Chim. Acta, 330, 136 (2002).
- Steed J. W., Atwood J. L., Supramolecular Chemistry, Wiley, Chichester (2000).
- Larsson K., Öhrström L., Inorg. Chim. Acta, 357, 657 (2004).
- BinnemansK., Lenaerts P., Driesen K., Görller-Walrand C., J. Mater. Chem., 14, 191 (2004).
- Lenaerts P., Storms A., Mullens J., D’Haen J. Görller-Walrand C., Binnemans K., Driesen K., Chem. Mater., 17, 5194 (2005).
- Frost C. G., Hartley J. P., Mini-Reviews in Organic Chemistry, 1 (2004).
- Allen C. L. Burel C., Williams J. M. J. Tetrahedron Let., 51, 2724 (2010).
- Li C., Zhang D., Han S., Liu X., Tang T., Lei B., Liu Z., Zhou C., Ann. N.Y. Acad. Sci., 1006, 104 (2003).
- Cansizoglu M. F., Engelken R., Seo H. W., Karabacak T., ACS Nano, 4, 733 (2010).
- Malyarick M. A., Petrosyants S. P., Ilyukhin A. B., Polyhedron, 11, 1067 (1992).
- Nan D., Naidong W., Zhenchao D., Shengzhi H., Jiegou Huaxue, 6, 145 (1987).
- Ahmadi R., Kalateh K., Abedi A., Amani V., Khavasi H. R., Acta Crystallogr., E64, m1306 (2008).
- Ilyukhin A. B., Malyarik M. A., Kristallografiya, 39, 439 (1994).
- Kalateh K., Ahmadi R., Ebadi A., Amani V., Khavasi H. R., Acta Crystallogr., E64, m1353 (2008).
- Abedi A., Safari A.R., Amani V., Z.Kristallogr. New Cryst. Struct., 227, 196 (2012).
- Shirvan S.A., Haydari Dezfuli S., Acta Crystallogr., E68, m1189 (2012).
- Shirvan S. A., Haydari Dezfuli S., Khazali F., Aghajeri M., Borsalani A., Acta Crystallogr., E68, m1448 (2012).
- Shirvan S. A., Haydari Dezfuli S., Golabi E., Gholamzadeh M. A., Acta Crystallogr., E68, m1327 (2012).
- Sheldrick G. M., SADABS. Bruker AXS, Madison, Wisconsin, USA (1998).
- Bruker, APEX2 software package,version 2.0-1, Bruker AXS Inc. Madison, Wisconsin, USA (2005).
- Sheldrick G. M., SHELXTL v. 5.1, Structure Determination Software Suite, Bruker AXS, Madison, Wisconsin, USA (1998).
- Mercury, Copyright Cambridge Crystallographic Data Center, 12 Union Road, Cambridge, CB2 1EZ, UK (2006).
- Amani V., Safari N., Khavasi H. R., Polyhedron, 26, 4257 (2007).
- Amani V., Safari N., Khavasi H. R., Mirzaei, P., Polyhedron, 26, 4908 (2007).
- Amani V., Safari N., Khavasi H. R., Akkurt, M., Polyhedron, 28, 3026 (2009).
- Alizadeh R., Amani V., Struct. Chem., 22, 1153 (2011).
- Alizadeh R., Amani V., Farshady A. A., Khavasi H.R. J. Coord. Chem., 63, 2122 (2010).
- Nakamoto K., Infrared and Raman Spectra of Inorganic and Coordination Compound Part B: Application in Coordination, Organometallic and Bioinorganic Chemistry, John Wiley and Sons Inc., New York (2009).
- Abedi A., Safari N., Amani V., Khavasi H. R., J. Coord. Chem., 65, 325 (2012).
- Abedi A., Amani V., Safari N., Main Group Chem., 11, 223 (2012).

This work is licensed under a Creative Commons Attribution 4.0 International License.









