Synthesis and Characterization of Cu(II), Ni(II) And Co(II) Coordination Compounds with Nitrogen and Oxygen Containing Schiff Base
Rajeshwar Rai1, Rajesh Ranjan Kumar1, Manoj Kumar1 and B. K. Rai*
Department of Chemistry, Darbhanga College of Engineering, Darbhanga, Bihar Department of Chemistry, L. N. T. College, B. R. A. Bihar University, Muzaffarpur
DOI : http://dx.doi.org/10.13005/ojc/300139
Article Received on :
Article Accepted on :
Article Published : 30 Mar 2014
Octahedral complexes has been designed and synthesized by a 2-propyl-thioquinozoline 4(3H) semicarbazone (PTQS) with Co(II), Ni(II) and Cu(II) metal ions. The complexes was characterized by molar mass, elemental analyses, IR electronic spectra, Molar conductivity and magnetic susceptibility assistance. On the above studies it was confirming that metal ions was coordinated with Carbonyl oxygen and azomethine nitrogen atom of semicarbazone moiety. The remaining valency of metal ions are satisfied by negative ions such as Cl–, Br–, I– and No3– ions.
KEYWORDS:PTQS/Semicarbazone/ Co(II); Ni(II) and Cu(II)/ Octahedral complex.
Download this article as:| Copy the following to cite this article: Rai R, Kumar R. R, Kumar M and Rai* K. B. Synthesis and Characterization of Cu(II), Ni(II) And Co(II) Coordination Compounds with Nitrogen and Oxygen Containing Schiff Base. Orient J Chem 2014;30(1). |
| Copy the following to cite this URL: Rai R, Kumar R. R, Kumar M and Rai* K. B. Synthesis and Characterization of Cu(II), Ni(II) And Co(II) Coordination Compounds with Nitrogen and Oxygen Containing Schiff Base. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2580 |
INTRODUCTION
In the last decade, there have been numerous attempts to produce transition metal complexes containing nitrogen and sulphur donor Schiff base ligands. Schiff bases and their metal complexes exhibited biological activity as antiviral1, antibiotics2 and anti-tumour agents3 due to presence of their specific moiety. The direct use of transition metal salts as antimicrobial agent cannot be recommended as they are very toxic to host human being. It has been proved from the survey of literature that the biologically active compound show greater activity when administered as metal complexes than as a free organic compounds. Semicarbazones are used as ligands in coordination chemistry and are biologically active compounds. Their complexation with different metal enhances the bioactivity of these molecules. These metal based complexes have attracted considerable interest in chemical and biological studies due to their potentially beneficial biocidal activity which may be attributed to formation of their chelates with metal ions5-10. Such promising biological potentials of Schiff base and its metal complexes as well as in continuation of our earlier research work11-13 in this field we have prompted us synthesized complexes of Co(II), Ni(II) and Cu(II) with Schiff base ligand, 2-propyl-thioquinazolin 4(3H) semicarbazone.
EXPERIMENTAL
All reagents, were of analytical reagent grade. The metal contents of all the complexes were analysed using standard procedure14. The infrared spectra of the ligand and metal complexes in the regions 200-4000 cm-1 were recorded on Perking Elmer -577 spectrophotometer. The electronic spectra were recorded on Cary-2390 spectrophotometer in the 10000-25000 cm-1 and magnetic susceptibility was measured using Gouy balance using mercury tetraisothiocyanto cobaltate as a calibrant. The molar conductivity was measured on Systronics Conductivity Meter model 303 using DMF as a solvent.
Preparation of the Ligand
A mixture of 2-propyl thioquinazoline 4(3H) one (5m mol) and semicarbazide hydrochloride (5m mol) was refluxed for 1 h. After cooling , the mixture was poured into distilled water (200 ml) white precipitate were filtered and recrystallised from ethanol. m.p.; 311 ± 10C.
Synthesis of Metal Complexes
The complexes were prepared by reacting respective halide/nitrate of Co(II), Cu(II) and Ni(II) in ethanoic medium with the ethanolic solution of the ligand, 2-propyl thioquinazoline 4(3H) Semicarbazone in molar ratio 1:2. The resulting reaction mixture was refluxed on water bath for 3-4h. The procedure carried out in each case was similar with slight variation of timing of reflux. On cooling solid coloured complexes separated out which were filtered, washed with ethanol, dried and recrystallised.
RESULTS AND DISCUSSION
Infrared Spectra
The mode of band formation in complexation between ligand and metal ions can be effectively determined by the Infrared spectral date (Table-2). The IR spectrum of the ligand PTQS exhibit strong and broad band at 3200 cm-1 assigned 15 to N-H. In the spectra of the complexes, this band is unaffected which indicates non-involvement of either primary amino or secondary amino group in the coordination. IR spectrum of the ligand exhibit strong and broad band at 1640 cm-1 assigned16 to C=O. In the spectra of the complexes, this band has shifted to a lower frequency region with slightly reduced intensity, The shift of the band and change in intensity proposes co-ordination of the carbonyl oxygen with metal ions. This is further confirmed on the basis of occurrence of a far IR region band of the complexes at 540-510 cm-1 assigned to M-O. The absorption bands around 1460 cm-1 are designated to the azomethine group18. In the complexes, the frequencies of azomethine groups appear mostly towards the lower region. The linkage of azomethine nitrogen with metal ion is further supported by the appearance of another far IR band at 440-405 cm-1 assigned19 to M-N. The above IR spectra data indicates that ligand coordinate to the metal through the carbonyl oxygen and azomethine nitrogen. The evidence of metal halogen linkage is confirmed by the appearance of a band in the region 320-270 cm-1 assigned20 to M-X (X=Cl, Br and I) The evidence of metal halogen linkage is further confirmed by the low value of molar conductance of the complexes on the range 1.8- 7.9 ohm-1 cm2 mol-1. Nitrate complexes show bands in the region 1340-1375 cm-1 and 800-825 cm-1 due to ionic nitrate and bands at 1025-1040 cm-1 can be assigned to coordinated nitrate group21. Appearance of bands at 1360-1440 cm-1 confirms the monodentate coordination mode of the nitrate group22.
Table-1 : Analytical, electronic and molar conductance measurements
|
Compounds (Colour) |
Molar Mass |
% Analysis found (calculated) |
meff B. M. |
Wm ohm-1 cm2 mol-1
|
DT oC |
l max electronic cm-1 |
|||
|
M |
C |
N |
H |
||||||
|
PTQS Colourless |
278 |
51.62 (51.79) |
25.04 (25.17) |
5.69 (5.75) |
|||||
|
[Co(PTQS)2Cl2] Brown |
685.93 |
8.50 (8.59) |
40.79 (41.98) |
20.28 (20.41) |
4.61 (4.66) |
5.1 |
3.3 |
1.89 |
20800 |
|
[Co(PTQS)2Br2] Brown |
774.75 |
7.51 (7.60) |
36.98 (37.17) |
17.96 (18.07) |
4.07 (4.13) |
4.96 |
3.1 |
193 |
20730 |
|
[Co(PTQS)2I2] Brown |
868.73 |
6.70 (6.78) |
32.97 (33.15) |
16.02 (16.11) |
3.64 (3.68) |
4.92 |
2.6 |
198 |
21080 |
|
[Co(PTQS)2(NO3)2] Brown |
738.93 |
7.89 (7.97) |
38.71 (38.97) |
18.82 (18.94) |
4.29 (4.33) |
4.99 |
1.8 |
201 |
20300 |
|
[Ni(PTQS)2Cl2] Yellowish red |
685.71 |
8.50 (8.56) |
41.83 (42.00) |
20.28 (20.41) |
4.60 (4.66) |
3.04 |
4.7 |
204 |
11600, 15400, 23100 |
|
[Ni(PTQS)2Br2] Yellowish red |
774.53 |
7.49 (7.58) |
36.96 (37.18) |
17.98 (18.07) |
4.08 (4.13) |
3.08 |
4.9 |
211 |
11200, 15100, 23600 |
|
[Ni(PTQS)2I2] Yellowish red |
868.51 |
6.69 (6.75) |
32.94 (33.16) |
15.97 (16.11) |
3.60 (3.68) |
3.02 |
5.3 |
217 |
11480, 15300, 22800 |
|
[Ni(PTQS)2(NO3)2] Yellowish red |
738.71 |
7.87 (7.94) |
38.70 (38.98) |
18.80 (18.95) |
4.28 (4.33) |
3.11 |
6.8 |
223 |
12060, 15600, 23100 |
|
[Cu(PTQS)2Cl2] Yellowish red |
690.54 |
9.13 (9.20) |
41.52 (41.70) |
20.14 (20.27) |
4.58 (4.63) |
1.98 |
7.3 |
179 |
13080, 18900 |
|
[Cu(PTQS)2Br2] Yellowish red |
779.36 |
8.08 (8.15) |
36.89 (36.95) |
17.85 (17.96) |
4.06 (4.10) |
1.93 |
7.7 |
186 |
13400, 18200 |
|
[Cu(PTQS)2(NO3)2] Yellowish red |
743.54 |
8.48 (8.54) |
38.60 (38.73) |
18.71 (18.82) |
4.25 (4.30) |
1.89 |
7.9 |
189 |
13380, 18600 |
Electronic Spectra and Magnetic Susceptibility of the Complex
The electronic23 spectra of all the complexes have been recorded in the region 10000-25000 cm-1. The electronic spectral and magnetic susceptibility24,25 data (Table-2) suggest octahedral geometry of the complexes.
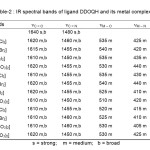 |
Table-2 : IR spectral bands of ligand DDOQH and its metal complexes. |
Conductivity measurement
Molar conductance values of the complexes of Co(II), Ni(II) and Cu(II) were found to be in the range 1.8-7.9 ohm-1 cm2 mol-1 in DMF proposes their non-electrolytic26 nature. The molar conductance values also supported the structure assigned on the basis of physiochemical and spectroscopic measurements.
CONCLUSIONS
Thus on the basis above studies it is concluded that the ligand PTQS acts in a bidentate manner and coordination is proposed through azomethine N and carbonyl oxygen of Semicarbazone moiety. The remaining centre of the metal ion is satisfied by negative ions such as Cl, Br, I or No3. The geometry of the Co(II), Cu(II) and Ni(II) complexes are proposed to be octahedral in nature as shown in Fig 1.
![Fig.1 [M(PTQS)2 ]](http://www.orientjchem.org/wp-content/uploads/2014/03/Vol30_No1_Synt_Rajesh_Fig1-150x150.jpg) |
Fig.1 [M(PTQS)2 ] Click here to View Figure |
M = Co(II), Ni(II); X = Cl–, Br–, I– and No3–
M = Cu(II); X = Cl–, Br– and No3–
REFERENCES
- Transconi. P, Albertini. R, Bonati, A. Agila, P.P. Dall, Lunghi P and Pinelli. S, Bionorg. Met. Chem., 8, 154(2000).
- Gulerman N.N., Rollas. S. and Erdeniz, H.J. Pharma., Sci., 26, 1(2011).
- Wang M, Wang L.F, Li.Y.Z, Li, Q.X, Xu, Zd and Qu. D.Q, Transition Met. Chem, 26, 307 (2001).
- Ramesh M., Chandrasekar K. B. and Reddy, K.H., Indian J. Chem.; Spectrum, Academic Publishers, Oxford (1996).
- Reyk D. Van, Sarel S. and Hunt N., Biochem, Pharmacol., 60, 581 (2000).
- Beraldo H. and Gambino D., Min. Rev. Chem.; 4, 31 (2004).
- Ainscough E. W., Brodie A. M., Denny W. A., Finaly G. J., Gothe S. A. and Ranford J. D., J. Inorg, BioChem.; 77, 125 (1999).
- Rollas S. and Kucukguzel S. G., Molecules, 12, 1910 (2007).
- Liberta A. E. and West D. X., Biometals, 5, 121 (1992).
- French F. A., Blanz E. J. Jr.; DoAmaral J. R. and French D. A., J. Mol. Chem.; 13, 1117 (1970).
- Rai B. K. and Rai Rashwar, Orient J. Chem., 20, 493, 601 (2004); Rai B. K., Rai Rajeshwar, Sahi Poonam and Rana Swati, Asian J. Chem.; 20, 143, 149 (2008).
- (i) Rai B.K., Vidyarthi S.N., Sinha Puja, Singh Vineeta and Kumar Sanjiv, Orient J. Chem.; 29, 271 (2013); (ii) Rai B. K., Kumar Sanjay, Anand Rahul and Pandey Ashok, Orient J. Chem.; 29, 655 (2013); (iii) Rai B. K., Singh Ravindra, Anand Puja, Singh Sunil Kumar and Amit, Orient J. Chem.; 29, 753 (2013); (iv) Rai B. K., Vidyarthi S. N., Prakash Om and Baluni Akhilesh, Orient J. Chem.; 29, 801 (2013); (v) Chaudhary Chitranjan Prasad, Sharma S. P., Rai C. L. and Rai B. K., Orient J. Chem.; 29, 1187 (2013); (vi) Rai B. K. and Kumari Rachana, Orient J. Chem., 29, 963 (2013); (vii) Rai B. K. and Kumar Arun, Orient J. Chem.; 29, 1187 (2013); (viii) Kumar Ajit, Yadav U. S. and Rai B. K., Orient J. Chem.; 29, 1203 (2013).
- Rai B.K. and Anand Puja, Asian J. Chem.; 25, 480 (2013); (ii) Rai B.K., Thakur Amrita and Divya, Asian J. Chem.; 25, 583 (2013); (iii) Rai B.K., Vidyarthi S.N. Kumari Punam, Kumari Suman and Singh Rajkishore, Asian J. Chem.; 25, 941 (2013); (iv) Rai B.K. and Kumar Arun, Asian J. Chem.; 25, 1169 (2013);
- Vogel A.I., A Textbook of Quantative Chemical Analysis revised by Bessett J. Denny R.C., Jeffery J.H. and Mendham J. ELBS, 5th Edn., London (1996).
- Rao C.N. and Venkataraghwan R., Can. J. Chem., 30, 974 (1974).
- Shradha L.N. and Ganorkar M.C. Indian J. Chem., 27A, 617 (1988).
- Chaube S.N. Shrivastava J.P. and Mishra L.K. Inorg. Chim. Acta, 23, 1 (1977).
- Agarwal R.K., Arora K., Polish, J. Chem.;. 67, 219 (1993).
- Singh N.K., Shrivastav A.K., Agarwal R.C. Indian J. Chem.; 22A, 704 (1984).
- Walker A and Ferraro J. P., J. Chem. Phys., 43, 2689 (1965).
- Rakowski M. C., Rycheck M. and Busch D. H., Ionrg. Chem., 14, 1194 (1975).
- Ferraro J.R., Low Frequency Vibration of Inorganic and Coordination Compounds, Plenum Press, New York.
- Lever, A.B.P., Electronic Spectroscopy, Elsevier, Amsterdam, 395 (1968).
- Figgis B.N., Introduction to Ligand Field, Wiely Eastern Ltd., New Delhi, 279 (1976).
- Carlin R.L., Van Dryneveldt A.J., Magnetic Properties of Transition Metal Compounds, Spriner Veralg, New York (1977).
- Kettle S.F.A. Coordination Compounds, Thomas Nelson and Sons, 168(1975).

This work is licensed under a Creative Commons Attribution 4.0 International License.









