Synthesis and Characterization of Bioactive Amphiphilic Graft Copolymers with Hydrophilic Poly (1, 3-Dioxolane) Side Chains
Boukar Mohammed1, 2 , Salmi Hassania1 and Ould Kada Seighir
1 Department of sciences, Faculty of sciences and technology, University of Bechar 08000. Algeria 2 Laboratory of Macromolecule Chemical- Physical, University of Oran, Department of Sciences, El Menouar Bp 1524 Oran 31000. Algeria
DOI : http://dx.doi.org/10.13005/ojc/300119
Article Received on :
Article Accepted on :
Article Published : 30 Mar 2014
2-oxypropylmethacrylat termined poly (1, 3 dioxolane) macromonomers having various chain lengths, were prepared by cationic polymerization using H2SO4 as initiator. The end group is introduced via the termination (end capping) using 2-hydroxypropylmethacrylate (2-HPMA). The amphiphilic graft copolymer is formed by the hydrophilic poly (1, 3- dioxolane) side chain and hydrophobic poly 2-hyèdroxypropylmethacrylate backbone with various chain lengths, were synthesized by free radical copolymerization of poly (1, 3 dioxolane) ω- oxypropylmethacrylate in the presence of 2- hydroxypropylmethacrylate using benzyle peroxide as initiator.
KEYWORDS:cationic polymerization; macromonomer; radical free copolymerization; amphiphatic; graft copolymers; surface tension; poly (1;3 dioxolane)
Download this article as:| Copy the following to cite this article: Mohammed B, Hassania S and Seighir K. O. Synthesis and Characterization of Bioactive Amphiphilic Graft Copolymers with Hydrophilic Poly (1, 3-Dioxolane) Side Chains. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Mohammed B, Hassania S and Seighir K. O. Synthesis and Characterization of Bioactive Amphiphilic Graft Copolymers with Hydrophilic Poly (1, 3-Dioxolane) Side Chains. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2544 |
Introduction
The graft copolymers have received attention recently [1-3], they have many important application [4- 10] in the polymer industry, mainly as surface modifiers for uses as coating, adhesives and dispersants; as compatbilizing agents in polymers blends, but also biomedical use. The amphiphilic graft copolymers are investigated widely because of their special physical and chemical properties and self assembly morphologies [11] in order to obtain amphiphilic graft copolymers with hydrophilic side chains of defined size, we have chosen a method using a precursory polymer[12- 15].
Experimental Section
Materials
The monomers 1, 3 dioxolane (DXL prolabo) were purified by vacuum distillation, sulfuric acid (H2SO4, prolabo). The free radical initiator employed for the polymerization was benzyl peroxide (Fluka) all solvents chloroform, dicloromethan, tetrahydrofurane and cyclohyxane, were purified by standard procedures.
Measurements
UV spectra were record on a Spectronic Gensys 5 spectrometry using chloroform as a solvent. FT, IR spectra were performed on a nocolet 520 by 4000- 400 cm-1range spectrophotometer, the 1H-NMR and 13C NMR spectra of the polymers were recorded using Brucker DPX 300 MHZ spectrometer at room temperature, tetramethylsilane (TMS) is the internal standard. Viscosities of the polymer solutions (0,1- 1g/dl were measured with a capillary viscosimeter (ubbelhode viscosimeter).
Synthesis of poly (1, 3- dioxolan) macromonomers
1, 3- dioxolane is dissolved in 10 ml of freshly distilled chloroforme and placed under nitrogen atmosphere in a three- necked flask equipped with a condenser and magnetic stirring. The 2-hydoxypropylmehacrylate is added to the reaction mixture, the polymerization is terminated by introducing dimethylamine. The precipate is filtered off, washed with ether and dried in vacuum. The synthesis of a macromonomer of 1, 3-dioxolane is shown in scheme below.
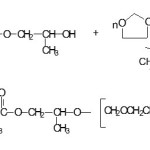 |
Scheme 1: Click here to View Scheme |
Table 1 list the experiment conditions of the reaction.
Table 1. Experimental conditions and yields of cationic polymerization of 1, 3- dioxolane initiated by H2SO4.
| Product | [DXL] | [2HPMA] | [H2 SO4] | [DXL]/ | Rendement |
| (mol/l) | (mol/l) | (mol/l) | [2HPMA] | ||
| BMD1 | 12,550 | 0,658 | 0.025 | 19,07 | 86% |
| BMD 2 | 13,127 | 0,327 | 0.688 | 40,14 | 73% |
| BMD3 | 12,550 | 0,344 | 0,125 | 36,48 | 78% |
| BMD4 | 12,550 | 0,172 | 0,3546 | 72,96 | 82% |
| BMD5 | 13,127 | 0,1635 | 0,456 | 80,28 | 88% |
preparation of the 1, 3- dioxolan graft copolymer
The polymerization is initiated by benzoyl peroxide . After 24 hours, the copolymer were precipitated into excess of cyclohexane in order to eliminate the poly(methylmethacrylate) homopolymer, dried under vacuum and dissolved in chloroform and precipitated again in water for eliminating the 1, 3- dioxolan monomer. The experimental condition is reported in Table 2.
Table 2. The experimental conditions of formation the copolymer 1, 3-dioxolane- g- 2- hydrpropylmethacrylate.
| [P]0 Mole/l | [ M ]0 mole/ l | [PB] mole/l | [M]0 / [P]0 |
| 0,124 | 0,07 | 1,3.10-1 | 9,66 |
| 0,124 | 0,401 | 0,061.10-1 | 3,23 |
Notes: [P]0: initial concentration of poly (1, 3- dioxolane) macromonomer calculated by UV analysis; [M]0: initial molar concentration of 2hydrxypropylmethacrylate comonomer; [PB]0: initial molar concentration on benzoyl peroxide initiator; Poly(DXL-g- 2HPMA); poly (1, 3-dioxolan graft poly methylmethacrylat), P(DXL- g- 2-HPMA): poly 1, 3 dioxolane-g- 2-hydroxypropylmethacrylate.
Results and Discussion
UV analysis of oxypropylmethacryloyle end chain
UV analysis is used; on one band (λ= 220 nm) to observe the fixation of chromophoric group oxypropylmethacrylate on the 1, 3- dioxolane end chain on the other hand to calculate number- average molecular weight Mn of the macromonomer. By assuming, that only one of the chromophoric groups is attached to end polymeric chain and by using molecular extinction coefficient of molecular model, in this case, the 2-hydrxypropylmethacrylate (2-HPMA), we can calculate the molecular weight of our samples. Thus, starting from a calibration curve representing the variation of the optical density according to the concentration of 2-HPMA in chloroforme. We have obtained a curve which follows BEER LMABERT’s low, which can calculate the molecular extinction coefficient of 2-HPMA taken as model (ε=8463 at λ= 220 nm) in chloroforme. The number average molecular weights calculated from UV analysis are listed in Table 3.
Table 3. Numbers of average molecular weights of 1, 3-dioxolane macromonomers calculate from UV analysis (ε= 8643, λ=220 nm, solvent: dichloromethane).[17]
| Product | théorical | UV |
| M1, | 1500 | 1500 |
| M2 | 2000 | 2000 |
| M3 | 2500 | 2500 |
| M4 | 3000 | 3000 |
Determination of the number- average molecular weight by viscosity
Viscosity measurements used to obtain the average molecular weights of polymers produced the Mark- Houwink equation was used to calculate the viscosity average molecular weight [16]. The values of Mn are presented in Table 4.
Table 4. Number average molecular weight Mn of 1, 3- dioxolane macromonomers determined by viscosity (K= 2,3 10-2, α=0,65, T= 25°C solvent: dicloromethane).
| macromonomer | Mn(viscosity) |
| M1 | 1600 |
| M2 | 2100 |
We notice that the values of the number- average molecular weight determined by UV analysis and by viscosimetry are relatively in accordance with those calculated theoretically. This results suggest that the contribution of transfer reactions could by much less important, which could mean that the reaction occurs, according to a “living form”.
Analysis FT-IR
The FT-IR spectra of 1, 3-dioxolane macromonomers (Figure 1) shows the most characteristic absorption at 1732 cm-1 corresponds to the carbonyl group, the peak at 2945 cm-1 due to the stretching vibration of methyl group in the end of chain, the peak at 1222 cm-1 is attributed to stretching vibration band of the double bond. The bands at 1082 cm-1are assigned to ester faction. The peak at 886 cm-1 and 580 cm-1corespond to bonding vibration, we notice the disappearance of characteristic band at 1630 cm-1 and 981 cm-1related to the double bondof themonomer and a new characteristicpeak appears at 1384 cm-1 due to the oxypropylmethacrylate double bond.
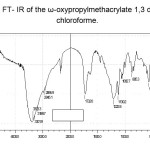 |
Figure 1. FT- IR of the ω-oxypropylmethacrylate 1,3 dioxolane in chloroforme. |
Analysis 1H-NMR
The 1H-NMR spectra of 1, 3- dioxolane macromonomer was record in CDCl3 solution using a DPX 300 Brucker spectrometer; the 1H-NMR spectra of macromonomer is shown in Figure 2.
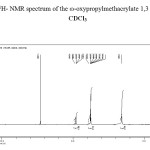 |
Figure 2. 1H- NMR spectrum of the ω-oxypropylmethacrylate 1,3 dioxolane in CDCl3 |
Characterization of copolymer by 13C-RMN.
The poy (1, 3- dioxolan)-g-PMMA was characterized by 13C-RMN, 13C-RMN analysis of poly (1, 3-dioxolan)-g-2HPMA confirms that the chain are copped with poly (2-HPMA) backbone. The 13C-RMN spectrum of the copolymer shows the signal with chemical shifts of about 38-42 ppm, 36- 30 ppm correspond to the –CH2 – carbon atms and those observed at 170- 178 ppm and 57 ppm are attributed respectively to carbonyl carbon and CH- carbon of oxypropylmethacrylate.
Determination of the surface tension of the copolymer poly (1, 3-dioxolan)-g-PMMA
Interestingly, the amphiphilic character of the poly (1, 3-dioxolan) in the graft copolymer, has been evidenced by the superficial tension measurements. Purposely; solution of poly(1, 3-dioxolan)-g-PMMA, in water, Figure 3 shows the logarithmic plot of superficial tension of the graft copolymer solution vs, concentration (expressed in g/l at 25°C, the superficial tension goes down from 63;3 N/m to 53,9 N/m, demonstrating the tensioactive proprieties of the new type of amphiphilic graft copolymer. The critical micelle concentration (CMC) has been determined from the intersection between the tangents drawn from higher concentration portion of the sigmoid plot and calculated as 10-4g/l.
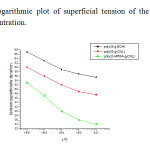 |
Figure 3. The logarithmic plot of superficial tension of the graft copolymer solution vs, concentration. Click here to View Figure |
Conclusions
The graft copolymer of poly (1, 3dioxolan)-g-MAA were prepared by radical copolymerization. Amphiphilic graft copolymers containing hydrophobic poly (2-hydroxypropylmethacrylate) backbone and hydrophilic poly (1, 3-dioxolan) side chain were synthesized by free radical copolymerization, using benzoylperoxide as initiator. The analysis by 1H NMR, 13C NMR, FTir and UV confirmed the sequence copolymer of poly (1,3- dioxolane)-g-P-2HPMA. These materials have potential applications in various fields and in particular with the aim of pharmacy and medicine.
References
- Tsukahara. Y; Mizuno. K, Segawa. A, Yamashata? Y study on the radical polymerization behavior of macromonomers. Macromolecules 1989, 22, 1546- 1552
- Neugebauer. D, zhang. Y, pakulaaaaa; T, Sheiko. S Matyjaszewky. K densly-grafted and double grafted POE burushers via ATPR ; a route to soft elastomers. Macromolecules. 2003, 36, 6746-6755.
- Debuigne. A, Caille. J. R, Willet. N, Jerome. R, synthesis of poly vinyl alcohol) containing block copolymers by combination of cobalt mediated radical polymerization and ATRP, macromolecules 2005, 38, 9488-9496.
- Mozali. M, Lacoste. J, Abadie. M. J. M, Synthese de copolymers styrene/isoprene/styrene α, ω- diepoxydes ; propriétés adésives. Eur, Polym, J, 1992, 28, 1241-1246.
- Eguiburu. J. L, Berridi. M. J. F, Roman.J. S, graft copolymers for biomedical application prepared by free radical polymerization of poly(lactide) macromonomers with vinyl and acrylic monomers, Polymer, 1996, 37, 3615- 3622.
- Soykan. C, Koskun. M, Ahmadazade. M, Ozdemi. E, phenacylmethacrylate and N- vinyl-2-pyrrolodone copolymers, shyntesis, characterization reactivity ratios. Pure and appl. Chem., A 2000 37 (9), 1089-1101.
- Coulembier. O, Degree . P, barbaud. C, Urén. P, Dubois. P, new amphiphilic graft copolymer based on poly(β- malic acid ; synthesis and characterization, polymer Bulletin, 2004, 52.
- Gao. Y, Li, S, Li. H, Wang. X, synthesis of syndiotactic polystyrene- graft poly(methacrylat and poly(styrene) and syndiotactic polystyrene- graft acatic poly(styrene) by atom transfert radical polymerization, Eurp. Poly. J, 2005, 41, 2329-2334.
- Li. Z, Li. P, Huang. J. synthesis and characterization of amphiphilic graft copolymer poly ethylene oxide)- graft- poly methacrylat) polymer, 2006, 47, 5791- 5798.
- Chun. Y, Chlu. Y, Jie Y, Shio. W. K, Hsien. X. C, Feng. C. C ; complicated phase behavior.
- Riess. G, micellization of block copolymers, Prog. Poly, sci. 2003, 28, 1107- 1170.
- Ranucci. E, Spagnoli. G, Sartore. L, Bigntti. F, Ferruti. P, Schiavon. O. synthesis and molecular weight charatcterization of end- functionalization of N- vinyl-2- pyrolidone oligomers. Macromol. Chem. Phys, 1995, 196, 763-774.
- Raccini. E, Tarabic. M, Alerston. A. new ester and lactone end functionalized N-vinyl- pyrolidone oligomers. Macromol. Chem. Phys, 2000, 201, 1219-1225.
- Garibi. H; palepu. R, Tiddy. G. J. T; Hall. D. G, Wyne. J; 1990. J. Chem. Soc. Commun.2; 115.
- H. Bendaikha, G. Clisson, A. Khoukh, J. François, S. Ould Kada. Synthesis and characterization of amphiphilic graft copolymers of macromonomers of (1, 3- dixolane) with methacrylate and styrene. e-polymer, 2009, 09, 9- 28.
- M. Fontanille, Y, Gnanou. Chimie et physic- chimie de polymère, Ed, Paris, Dunod, 2002, Chap. 6. M. Boukar, mémoire de magister, université d’Oran Es-Senia, 2000, Algerie

This work is licensed under a Creative Commons Attribution 4.0 International License.









