Structure of Starch-Ionic Surfactant Complexes Studied by Ternary Phase, XRD and Scanning Electron Microscopy
Md. Mohsin Hossain, Md. Ibrahim H. Mondal and Md. RaihanSharif
Polymer and Textile Research Lab, Department of Applied Chemistry and Chemical Engineering, Rajshahi University, Bangladesh
DOI : http://dx.doi.org/10.13005/ojc/300109
Article Received on : January 19, 2014
Article Accepted on : March 05, 2014
Article Published : 13 Mar 2014
Thestructureoflyotropicliquidcrystallineorgel like phasesformedin starch/surfactant (cationicoranionic)/waterternaryphasesystemsinthetemperature rangeof 25-85oChas beeninvestigatedbyXRD,SEMandFT-IRtechniques.The ionicsurfactantswerecetyltrimethylammonium bromide(CTAB),sodiumdodecylsulfate (SDS).Thephaseswereinequilibriumwithaqueoussolutionsat 60°Candcontained15-25wt%starchand10-30wt%surfactant,dependingonthechargedensityof thestarchand thechainlengthofthesurfactant.Thephasesconsistofstarch/surfactant aggregatesarranged in long-range structures similartolyotropicmesophasesformedbythepureionicsurfactantsalone, butthey separatefromaqueous solutionsat muchlowersurfactant concentrations.Whenthechargedensityofthe starchislow orthe surfactanthydrocarbonchainisshort,the characteristiccubicor hexagonal phasesare formed.As expected,the formation of lamellar phaseis promotedbyincreasingtheseparameters.Temperature affectsthestabilityofthe phasesand theirstructure.Athightemperaturesthelong-rangeorder breaks down,and thephasesareakin toconcentratedmicellarstarch/ionic surfactantsolutions.
KEYWORDS:Starch; ionic surfactant; starch-ionic surfactant complexes; ternary phase diagram
Download this article as:| Copy the following to cite this article: Hossain M. M, Mondal M. I. H, Sharif M. R. Structure of Starch-Ionic Surfactant Complexes Studied by Ternary Phase, XRD and Scanning Electron Microscopy. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Hossain M. M, Mondal M. I. H, Sharif M. R. Structure of Starch-Ionic Surfactant Complexes Studied by Ternary Phase, XRD and Scanning Electron Microscopy. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2324 |
INTRODUCTION
The research understanding of polymer/surfactant interactions in aqueous solution has been summarized in several reviews1-4Many investigations have focused on aggregate structure in dilute solution, i.e., system in which phase separation does not occur. The general picture emerging from these studies is that in dilute solution the surfactant molecules adsorb to polymer chains as micellar or micelle-like clusters. A general phenomenon in system of polyelectrolyte and oppositely charged surfactant is that complexes of these components separate as a water-swollen phase in equilibrium with very dilute aqueous solution. Generally, the rich phase behavior of surfactants in water is also characteristic of polyelectrolyte-surfactant complexes in contact with water. Harada and Nozakura5 described the formation of layered structures in polyvinyl sulfate (PVS)/cetyltrimethylammonium bromide and 1-4-ionene/sodium dodecyl sulfate (SDS) systems. Moreextensivestudiesofx, y-ionene/SDS andPVS/n-alkylpyridiniumsurfactantssystemshaverecentlybeen reportedby Chenetal.6,Kimetal.7,Kabanovetal.8andKhandurinaet al.9.Demboetal.10andRogachevaetal.11observed the complexlamellar structures of sodium poly(acrylate)gel and alkyltrimethylammonium bromides.Hanssonand Langmuir12foundthatacubicphasewasformedby sodiumpoly-(acrylate) anddodecyltrimethylammonium bromide.Ilektiet al.13studiedsodium poly(acrylate)/cetyltrimethylammoniumbromidecomplexesandfound thattheyformhexagonalstructures,andthattheioniccompositionsof theconcentrated anddilutephaseshavesignificanteffectonthephase behavior.Kosmellaetal.14andRuppeltetal.15foundthattheintercalationofsodiumpoly(acrylate)into mesophasesformedbyalkyltrimethylammoniumbromidesinduced somedisordering, but didnotsubstantiallyaffect the phasestructures.Very recentlyZhouet al.16observed long-rangeorderincomplexesofpoly(sodiummethacrylate-co-N-isopropyl-acrylamide)and poly(styrenesulfonate) withtetradecylhexadecyl, ordodecyltrimethylammoniumbromide.Zhouetal.17discussedacharacteristicofthe complexesin this investigationisthattheycontainaveryhydrophilic carbohydratepolymer associatedwithoppositelychargedsurfactants.Thus,we expectthe predominant
EXPERIMENTAL
Materials
ThisproductwaspurchasedfromUNI-CHEM,China. Thedegree ofsubstitution (DS)was 0.80.Thestarchwasdissolvedbyheatingthe starch/watermixtureinan autoclaveat120oCfor 30 min. Allsolutionswerepreparedatleast24hbeforemeasurements wereperformed.
Thewaterwasionexchangedanddistilled.Itsconductivity andreducedviscositywere2.0µsand 4.0 dm3/mol,and itssurfacetensionwas71.5±0.5 mN/mat 30oC.Surfactants and all otherchemicalswere of analyticalgradeandwereusedwithoutfurtherpurification.
Sample preparation for SEM
Weighted amounts of starch, surfactants and distilled water were added to tightly closed test tubes. The tubes were equilibrated by continuously turning them over in a thermostat at 333K (for starch/SDS or CTAB the temperature was 298K) for 7 days. This resulted in the formation as a complex phase dispersed in aqueous solution. The charge neutrality of the complexes was verified by measuring the electrophoretic mobility of the dispersed particles. The solution and complex phases were separated by centrifugation for 30 min at 1600g. After centrifugation, samples were again allowed to equilibrate at the same temperature for 7 days. Then the phases were separated by careful decantation of the solution. The dry content of the complex phase was determined by weighing. Then the sample was analyzed by Scanning Electron Microgram.
Sample preparation for FT-IR
An FT-IR spectrum of dried sample was obtained with SHIMADZU IR-470 infra-red spectrophotometer. After drying the sample with KBrpellate at room temperature, it was again dried at 60oC for 1 h under reduced pressure and then its individual IR spectra were measured. In case of sample crystalline (Tween-20) IR spactra was measured on the Teflon plate at controlled temperature and pressure.
Sample preparation for XRD
For the preparation of starch sample, the dried sample is saturated with water by stirring repeatedly with a glass rod. This step is performed by 2g of starch in 100 mL of hot distilled water and stirred at room temperature for 1 h, centrifuging the suspension and decanting the supernatant solutions. This process is repeated three times. Then the starch solution is dried in oven at temperature 80oC. After 2 h drying, the powder sample was kept in a sealed bottle. The syntheses of cationic surfactants were undertaken by the following procedure: 2g of cetyltrimethylammonium bromide (CTAB) was first dispersed in 100 mL of deionized water andthen under mechanical stirring for about 1h. A pre-dissolved starch solution of same amount was slowly added to the suspension at 80oC. The reaction mixtures were stirred for 1 h at 80oC using mechanical stirring. After proper drying the powder sample was kept in a sealed bottle and stored in a vacuum desiccator. Starch powders to be used for X-ray diffraction (XRD) measurements were equilibrated in a desiccators containing saturated solutions of K2CO3 at 20-22oC. Under these conditions, the relative humidity (RH) at 20oC was 44% and the final water content of potato starches was 15%. Wet starch powder (from potato) for XRD measurements was produced by first equilibrating the starch excess water. The starch suspension was then centrifuged and the supernatant removed. The starch precipitate appeared as a hard wet powder that was slightly moremoist at the top. This moisture was dried with tissue paper. The wet starch powder had a water content of 49%. It was apparent that the proportion of water was slightly overestimated, because the precipitated starch granules would have a small amount of free space between them, which would be filled with free water. This overestimation, however, can be considered to be very small as the granules in the precipitate were closely packed. Since the water content within the crystallites is fixed near about 24% the proportion of water in the amorphous part of starch can be estimated at 55-60%. It was assumed that total crystallinity of starch and complexes crystallinity were within the range 25-40% and 40/60-50/50 respectively.
Samplepreparationforternaryphase
Anequilateraltriangularwoodenframeconsistsof228holes.Forthedevelopmentofternaryphasediagrams,thesamplecomponentsweretakenintothetest tubebyvarying compositioninsuchawaythatthetotalcompositionremains100%.The componentswere addedbyvaryingweightbyvolumes.Thesampleswerepreparedbyvarying 5%composition oftwocomponentssimultaneouslykeepingthethirdcomponentconstantalternativelyinatesttube.Theopen endofthetesttubewasthenclosedwithrubbercorkinsuchawaythatthevapourcomesoutandenters intothetesttube,here thecorkreactwiththesample. Thesampleswerethen shakenforwellmixingofthe componentsandplacedintothediagramaccordingtothecompositionandmark.Theopenendofthetest tubewasthenclosed tightlywithcork,sothatthisremainsnoleakageorthelowerpartofthecorkdidnottouchthesample solution and thesesampleswerethenlefttoequilibrateinathermostatboxat30°Cforatleasttendaysand shaken fromtimetotime.Theequilibrium wasestablishedwithinthis period.
Scannning Electron Microscopic Examination
Procedure for sample determination
ScanningElectronMicroscope(SEM)ofpotatostarch,surfactant sampleandstarch-surfactant complexessampleswereexaminedaccordingtoAtichokudomchaietal.26.Allthe sampleswere driedbeforeexamined.Theexcesssample was removed andplacein afine coaterforgoldcoatingfor150sec. Thecoatedsample was placedina samplechamberintheSEM. The sample was examinedatmagnificationof2,500×and 6,000×withtheacceleratingvoltage of10 kV.
SEM analysis procedure
AllsampleswereanalyzedusingaScanningElectronMicroscopewithanOxfordINCA X-sightEDXA(EnergyDispersiveX-RayAnalysissystem).The SEMisahigh-performance,Scanning ElectronMicroscopywithahighresolution of3.0 nm. Thelowvacuummodeallowsforobservation of specimenswhichcannotbeviewedathighvacuum duetoexcessivewatercontentorduetoanon- conductivesurface.SEManalysiscanbe used toexaminesurfacefeatures,texturesand particlesthat aretoosmallto seewithstandardopticalmicroscopes.
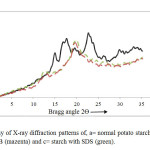 |
Fig.1 Calligraphy of X-ray diffraction patterns of, a= normal potato starch (black), b=starch with CTAB (mazenta) and c= starch with SDS (green). Click here to View Figure |
Effectofaddingsurfactant(SDS,CTAB)onthestarchstructure
XRDwasusedtoexaminethetypeandextentofcomplexityresultingfromcoolingofthestarch paste inthepresenceofSDSorCTAB.Increased relativeintensity wouldgenerallyreflecta greaterdegreeofcomplexity.Withoutsurfactants,controlstarchshowedatypicalA-typepattern27(Fig.1a). With theadditionofeitherof thesurfactants,potatostarchexhibited atypicalV-typepatternafterheatingandcooling (Figs.1band1c).Similar result has been reportedbyGhiasietal.28.WhenTangand Copeland29foundthatmixedstearicacid andwheatstarchhad peaksat 2θ= 7.4o, 12.7o and 19.8o. Similar V-type pattern was produced for starch with SDS in the present study. Results of the present study found that patterns of starch/CTAB had slightly greater relative intensity than did the patterns of starch plus SDS supported by Copeland et al.30In the starch systems, XRD patterns in the presence of either surfactant were identical with respect to the type of crystalline structure. Like potato starch, diffraction patterns of SDS plus either starch (Fig. 1b) had a slightly greater intensity than did the diffraction patterns of either starch plus SDS (Fig. 1c). Unlike potato starch, diffraction peaks were not as prominent with either CTAB.With the addition of surfactants, potato starch and wheat flour produced a V-type diffraction pattern, while native wheat starch and flour had a typical A-type pattern similar to results reported by Abdel-Aalet al.31
RESULTS ANDDISCUSSION
When starchwasheatedinexcesswaterat75and95oC,thesolublepartwasremovedandthe insolublepartwasfreeze-dried. X-raydiffraction patternsoftheinsolubleresiduesarepresentedin Fig. 1.Thesampleheatedat75oC (Fig.1b)showedalowdegreeofcrystallinity, andthesample heatedat95oC (Fig.1a)wasessentiallynoncrystallinewhichGhiasietal.28showedat 75oC only5.9% ofthestarch,mainly foramylose.Therefore,atthattemperature,even themajorpartoftheamylosewasstillassociatedwiththegranules andtheorganizationofthestarch granulehadchanged.Considerablecontroversy existsconcerningwhichcomponentsamyloseor amylopectinis responsibleforthecrystallinityofstarch32-35. Accordingtopolymer physics,crystallities canoccurwhenever chainsegmentshappentobeparallelinlatticepositions. Therefore,starchcrystallitiescanformthroughhydrogenbondingbetweenlinear fractionand segmentsofthebranchedfraction whichwassupportedbyCaesarandCushing36[1971].X-ray diffractionpatternsoftheinsolubleresidueofthestarchobtainedfromheatedstarchtodifferent temperatures withSDSisshownin theFig.1c. Withoneexception (95oC),thesampleallshowedthe surfactant-amylosecomplexpattern,asindicatedbythestrongd-spacingat4.4and6.8Aoat95oC, thestarch-SDS complexwasnolongerobserved.Loss ofthecomplexat95oChelpsexplainwhy SDSdidnotpreventleachingofsolublefromstarchathightemperatures.Ghiasietal.28andGrayandSchoch37foundthatSDSwasnoteffectiveindecreasing solubility of starchesattemperaturesof85oCandabove.X-raydiffractionpatternsof solubleisolatedfromstarch andsurfactanttreatedstarchheated at95oCareshownin Fig.1c.X-raydiffractionpatternswere primarily V-hydratecomplex, asindicated bytheird-spacingsmarkedbyZobel23. The existence ofamyloseasaV-hydrate complexhasbeenreportedbyYamashita38.Original XRDFigs. 2, 3and4hasbeen given belowforbetter understandingstarch,SDS,CTABcomplexes asvariationofconcentration.
 |
Fig.2 OnlyStarchXRDof2q (90o) Click here to View Figure |
SEM of magnification 100 times in area of From Figs. 5 and 6, It is seen that the surface of SEM is homogeneous butitisclearedto identifythat thereactedstarch-SDScomplexes(Fig.7) hadalot ofruptured surfacesinthe magnificationarea. Hereitwas alsoidentified that thesmoothersurface existsin thestarchandSDSSEM images only. So itisclearlydecidedthatobviouslyinteractionmayoccurredinthe starch-SDScomplexes.so, in complexesthereisachangein structuralconfigurationnot onlythisin XRDimages fromFigs.3 and4comparisonwithonlystarchmorphology thechangesareobtainfromherethatcrystalinityvariesfromdifferentanglesandintensityanalysis.
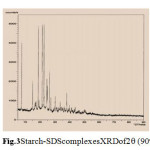 |
Fig.3 Starch-SDScomplexesXRDof2q (90o) Click here to View Figure |
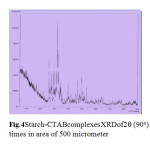 |
Fig.4 Starch-CTABcomplexesXRDof2q (90o) times in area of 500 micrometer Click here to View Figure |
 |
Fig.5 OnlyStarchSEMofmagnification100 Click here to View Figure |
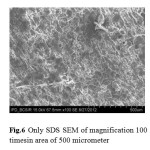 |
Fig.6 Only SDS SEM of magnification 100 imesin area of 500 micrometer Click here to View Figure |
 |
Fig.7 Starch (amylose)-SDS complexes reacted timesin area of 500 micrometer Click here to View Figure |
magnificationarea. Hereitwas alsoidentified that thesmoothersurface existsin thestarchandSDSSEM images only. Soitisclearlydecidedthatobviouslyinteractionmayoccurredinthe starch-SDScomplexes.so, in complexesthereisachangein structuralconfigurationnot onlythisin XRDimages fromFigs.3 and4comparisonwithonlystarchmorphology thechangesareobtainfromherethatcrystalinityvariesfromdifferentanglesandintensityanalysis.
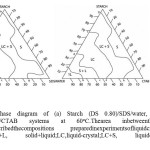 |
Fig.9 Ternary phase diagram of (a) Starch (DS 0.80)/SDS/water, (b) Starch (DS 0.80)/water/CTAB systems at 60oC.Thearea inbetweenthepointsdcefofboth Figuresdescribedthecompositions preparedinexperimentsofliquidcrystal.Here is 1L,liquid;S+L, solid+liquid;LC,liquid-crystal;LC+S, liquidcrystal+solid;andS, solid. Click here to View Figure |
Ternary phase diagram of (a) Starch (DS 0.80)/SDS/water, (b) Starch (DS0.80)/water/CTAB systems at 60oC.Thearea inbetweenthepointsdcefofboth Figuresdescribedthecompositions preparedinexperimentsofliquidcrystal.Here i 1L,liquid;S+L, solid+liquid;LC,liquid-crystal;LC+S, liquidcrystal+solid;and solid. Insummary, itcan beconcludedthatliquidcrystallinephasesareformedinthe
same sequenceandwithsimilarstructuresinthepolyelectrolyte/surfactantsystem as inpure binarysurfactant/watersystems.Theorderof thephasesisasafunctionofincreasing hydrocarbonchainlengthofthesurfactant:unorderedmicellar,cubic,hexagonal,cubic, andlamellar. Increasingthe temperatureanddecreasingthe chargedensityof the polymerhassimilareffect, but in this case order ofthephasesistheopposite:lamellar, cubic,hexagonal,cubic,andmicellarsolution.However, theconcentrationofsurfactantin the polyelectrolyte-containingphasesissubstantiallylowerthaninpuresurfactant system,andtheyalsoprecipitate fromsolutionscontainingmuchlesssurfactantthan
inthebinarysystem;i.e.,thetwo-phaseregionsbetweenliquidcrystallinephase and solutionareverywide.Thisshowsthattheeffectofaddingpolyelectrolyte isnotonlyan increaseintheionicstrength butprobablyalso anincrease indirectbindingofthe polyelectrolytetotheaggregate surfaces,which reducestheelectrostatic repulsion between theaggregates.Thisnotionisfurthersubstantiatedbythediscussionbelow. Thestructuralforce,duetothecollisionsbetweenthemoltenhydrocarbonchains,creates anoutwardpressure,tendingtoexpandthehydrocarbonmoiety.This is clearly reflectedinthetemperaturedependenceofthecurvatureoftheaggregatestructuresboth in puresurfactant/waterand starch/surfactant/watersystems.
Atthe polar/non-polarinterface,hydrophobicinteractionscreateaninwardpressure thattendstoreducewater-oilcontact. Intheheadgroupregiontherearesteric, electrostatic, andhydrationforces,which areexpectedto createanet positive outwardpressure.Whentheoutwardpressureinthechainregionishighthe monolayer islikelytobendtowardwater.Onbasis ofpresentlyavailableresults,theimportance of hydrophobic,stericand hydration forcesinthestarch/surfactant/watersystemsare difficulttoassess. However,thestronginteractionbetweenthe sulfateandCTAB groupsin thestarch/SDS complexesleadstoformationofaggregates withlower curvature than in corresponding starch/CTAB systems, which indicates that reduction of hydrophobic interactions and salvation in the surface plays a role. Whentheionic strength increases,thephaseboundariesofthelyotropicliquidcrystallinephases move towardlower surfactantconcentrations.They also shift to higher temperatures, indicatingincreasedstabilityof thesurfactantaggregates.However,this effectis muchweakerthantheeffectofaddingapolyelectrolyte.Forinstance,thephasediagram oftheNaPal/NaCl/watersystem.Laughlin39showsthatadditionofupto5wt% ofNaCltoa 1 wt% NaPalsolution doesnotcauseany phase changes.In our investigation,the initialSDSconcentrationofthestarch/SDSsamplewas1.06wt% andthestarchconcentration was 5wt%. The estimatedeffect ofstarchontheionic strengthcorrespondsapproximatelyto0.1wt%ofadded SDS.Thisindicatesveryclearly thattheadditionofapolyelectrolytehasamuchstrongereffectthantheadditionof asimpleelectrolyte.
CONCLUSION
Though, starch itself surface inactive but when it is added to ionic surfactants as a soap filler the physical properties of polymeric starch, such as surface tension, reduced viscosity, electric conductance etc. have been changed. It may occurred due to the formation of intermolecular H-bonding through polar part of ionic surfactants (CTAB, SDS) and H-atom from hydroxyl radical of amylase part of starch molecule, whereas non ionic surfactant (Tween-20) is absent. The fact is supported by XRD, SEM and FT-IR instrumental analyses.
REFERENCES
- Hayakawa,K. andKwak,J.C.T.,In CationicSurfactants (2nded.),Rubingh,N.D., Holland,P. M., (Eds.),MarcelDekker:NewYork, 1991, pp.189-248.
- Robb,I. D.,InAnionicSurfactants–PhysicalChemistryofSurfactant Action, Lucassen-Reynders,E. (Ed.),MarcelDekker: NewYork, 1981, pp.109-142.
- Saito,S.,InNonionicSurfactants,Schick,M.J. (Ed.)MarcelDekker: NewYork, 1991, pp.881-926.
- Goddard,E. D., Ananthapadmanadhan,K. P. (Eds.),InteractionsofSurfactants with Polymersand Proteins,CRCPress: BocaRaton,FL,1993,pp.1-427.
- Harada,A.,Nozakura,S.,Polym.Bull.,11,175-178, 1984.
- Chen,L.,Yu,S.,Kagami,Y., Gong,J.,Osada,Y.,Macromolecules,31,787-794, 1998.
- Kim,R.,Ishizawa,M.,Gong,J., Osada,Y.,J.Polym.Sci. A, 37,635-644, 1999.
- Kabanov,V.A.,Bakeev,K.N.,Yang, M.S.,MacKnight,W.J.,Zezin, A. B., Macromolecules,27,300, 1994.
- Khandurina,Y.V.,Rogacheva,V.B.,Zezin, A.B.,Kabanov,V.A., J.Polymer. Sci., 36,184-188, 1994.
- Dembo, A.T.,Khandurina,Y.V., Rogacheva,V.B.,Zezin,A.B.,Kabanov,V. A., J.Polymer. Sci. 36,189-194, 1994.
- Rogacheva, V.B.,Khandurina,Y.V.,Zezin, A.B.,KabanovV.A., J.Polymer. Sci., 36, 195-199, 1994.
- Hansson,P.,Langmuir,14,4059-4064, 1998
- Ilekti,P.,Piculell,L., Tounilhac,F., Cabane,B., J. Phys. Chem.B, 102,344- 351, 1998.
- Kosmella,S.,Ko¨tz,J.,Friberg,S.E.,MacKay, R., Colloids Surf.A,112,227- 231, 1996.
- Ruppelt,D.,Ko¨tz,J.,Jaeger,W.,Friberg,S.E.,MacKay, R.,Langmuir,13, 3316-3319, 1997.
- Zhou,S.,Burger,C.,Yeh,F.,Chu,B.,Macromolecules, 31,8157-8163, 1998.
- Zhou,S.,Yeh,F.,Burger,C.,Chu,B.,J.Phys. Chem. B.,103,2107-2112, 1999.
- Merta,J.,Stenius,P.,ColloidsSurf. A: PhysicochemAsp.,122,243-255, 1997.
- Goddard,E.D.,Hannan, R.B.,J.Colloid Interface Sci., 55,73-79, 1976.
- Goddard,E.D.,Hannan,R.B.,J.Am.OilChem. Soc., 54,561-566, 1977.
- Leharman, L., The nature of the fatty acid associated with starch. The adsorption of palmitic acid by potato and defatted corn and rice starches, J. Am. Chem. Soc.,64, 2144, 1942.
- Whistler, R.L., and Hilburt, G.E., Extraction of fatty substance from starch, J. Am. Chem. Soc.,66, 1721, 1944.
- Zobel,H. F.,X-rayanalysisofstarchgranules.InMethodsinCarbo-hydrateChemistry (Vol. 4),Whistler, R. L. (ed.),AcademicPress:NewYork,NY, 1964, pp.109–113.
- Osman, E. M., Leith, S. J., andFles, M., Complexesofamylasewithsurfactants,Cereal Chem.,38, 449, 1961.
- Krog, N. and Nybo-Jensen, B., Interaction of monoglycerides in different physical states with amylase and their anti-firming effects in bread, J. Food Technol.,5, 77, 1970.
- Atichokudomchai,N., Shobsngob,S. andVaravinit, S., Morphologicalpropertiesofacid- modified tapiocastarch,Starch/ Starke,52,283-289, 2000.
- Blazek,J.and,Copeland,L.,Theeffectofmonopalmitinonpastingproperties of wheat starches withvaryingamylosecontent, CarbohydratePolymers, 78, 131- 133, 2009.
- Ghiasi,K.,Varriano-Marston, E.andHoseney,R. C.,Gelatinization ofwheatstarchII: Starch- Surfactantinteraction,CerealChemistry,59(2),86-88, 1982.
- Tang,M.C.andCopeland,L.,Analysisofcomplexesbetweenlipidsand wheatstarch, CarbohydratePolymers,67, 80-85, 2007.
- Copeland,L.,Blazek,J.,Salman,H.and Tang,M. C.,Formandfunctionalityof starch, FoodHydrocolloids, 23,1527-1534, 2009.
- Abdel-Aal,E. S. M., Hucl,P.,Chibbar,R. N., Han,H. L.,andDemeke,T., Physiochemical andstructuralcharacteristicsoffloursandstarchesfromwaxy andnonwaxywheats, CerealChemistry,79(3), 458-464, 2002.
- Banks,W.andGreenwood,C.T., In StarchandItsComponents,Halstead press: Division of John Wileyand Sons, Inc., New York, 1975, pp. 67–110.
- Kainuma, K.andFrench, D.,Nageliamylodextrinanditsrelationshiptostarchgranule structure. I.Preparationandpropertiesofamylodextrinsfromvariousstarchtypes, Biopolymers, 10, 1673, 1971.
- Robin, J. P., Mercier, C., Charbonniere, R.andGuilbot, A., Insolubleresiduesfrom prolongedandtreatmentof potatostarch,CerealChemistry,51, 389, 1974.
- Sarko,A. andWu, H. C. H.,ThecrystalstructuresofA-B-andCpolymorphosof amylose, Starch/ Starke, 30, 73-78, 1978.
- Caesar,G. V., andCushing, M. L., Thestarchmolecule,Journalofphysicalchemistry, 45, 776, 1971.
- Gray, V. M., and Schoch, J. J., Effects of surfactants and fatty adjuncts on the swelling and solubilization on granular, Starch/ Starke, 14, 239, 1962.
- Yamashita, Y.,SinglecrystalsofamyloseVcomplexes,JournalofpolymerScience,A3, 3251, 1965.
- Laughlin,R.G.,TheAqueousPhaseBehaviourofSurfactants,AcademicPress:NewYork,1994, p.588-597.

This work is licensed under a Creative Commons Attribution 4.0 International License.









