Reductive Amination of Aldehydes by NaBH4 in the Presence of NaH2PO4.H2O
Hojjat Arefi and Davood Setamdideh*
Department of Chemistry, College of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, Iran,
DOI : http://dx.doi.org/10.13005/ojc/300138
Article Received on :
Article Accepted on :
Article Published : 03 Mar 2014
Thereductive aminationofa varietyof aldehydes withanilines has been carried out by NaBH4/NaH2PO4.H2Oas new reducing systemswithin 55-100 min in THFunder reflux conditionsin high to excellent yields of products (85-92%).
KEYWORDS:NaBH4; NaH2PO4.H2O; Reductive amination; Aldehydes; Amin
Download this article as:| Copy the following to cite this article: Arefi D, Setamdideh D. Reductive Amination of Aldehydes by NaBH4 in the Presence of NaH2PO4.H2O. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Arefi D, Setamdideh D. Reductive Amination of Aldehydes by NaBH4 in the Presence of NaH2PO4.H2O. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2267 |
Introduction
The reduction of nitro, cyano, azide, carboxamide compounds is common routes for the synthesis of amines. Also, this goal has been achieved by the alkylation of amines. These methods for the preparation of secondary amines are often problemssuch as harsh reaction conditions, overalkylation, low chemical selectivity and generally poor yields. Other approach is reductive amination reaction in a single operationi.ereductive amination. The reductive aminationhas been carried out by sodium borohydride under different reducing system such as: NaBH4/cellulose sulfuric Acid/EtOH1, NaBH4-amberlyst15 2, NaBH4-silica chloride 3, NaBH4-silica-gel-supported sulfuric acid 4, NaBH4-H3PW12O40 5, NaBH4/guanidine hydrochloride /H2O 6, NaBH4/Bronsted acidic ionic liquid (1-butyl-3-methyl imidazoliumtetrafluoroborate [(BMIm)BF4]) 7, NaBH4 or LiAlH4/LiClO4/diethyl ether 8 NaBH4-PhCO2H 9, NaBH4-NiCl210, Ti(O-i-Pr)4-NaBH411, NaBH4-wet-clay-microwave 12, NaBH4/Mg(ClO4)213, NaBH4/B(OH)3 or Al(OH)3 14 NaBH4/Ga(OH)315.In continuing our efforts for the development of new reducing systems 16-26,in this context, we have carried outthe reductive amination reaction of aldehydes with anilinesby NaBH4/NaH2PO4.H2O system in THF.
Results and Discussions
The model reaction has been selected by reductive amination of benzaldehyde with aniline. This reaction was performedwith different molar ratio of the benzaldehyde/aniline/NaH2PO4.H2O/NaBH4in different solvents for the selection of appropriate conditions. Experiments have been shown that using 1eq. of NaH2PO4.H2Oin THF (3 mL) under reflux conditionsis the best conditions to complete the reductive amination of benzaldehye (1 mmol) and aniline (1 mmol) to N-benzylaniline. The reductive amination completes within 55 min with 92% yields of product as shown in
scheme 1.
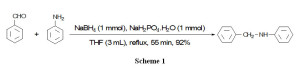 |
Schem 1: Click here to View Schem |
By using the various structurally different aldehydes and anilines, the efficiency of this protocol was further examined. Experiments have been shown the correspondingsecondary amines were obtained in excellent yields (85-92%) within 55-100 min as shown in Table 1. The influence of NaH2PO4.H2O is not clear but we observed sodium borohydride slowly is liberated hydrogen gas in situ in the presence of NaH2PO4.H2O. Consequently, the generated molecular hydrogen combines with more easily hydride attack, thus accelerates the rate of reduction reaction.
| Table 1. Reductive Amination of Aldehydes (1 mmol) with Anlines (1 mmol) by NaBH4 (1 mmol) in the presence of NaH2PO4.H2O(1 mmol) in THF (3 mL) under reflux conditions. | |||||
|
Entry |
Aldehydes Anilines Products |
Time/min |
Yielda/% |
||
|
1 |
benzaldehyde |
aniline |
N-benzylaniline |
55 |
92 |
|
2 |
benzaldehyde |
4-bromoaniline |
N-benzyl-4-bromoaniline |
65 |
87 |
|
3 |
benzaldehyde |
4-methylaniline |
N-benzyl-4-methylaniline |
60 |
89 |
|
4 |
benzaldehyde |
4-nitroaniline |
N-benzyl-4-nitroaniline |
65 |
88 |
|
5 |
4-bromobenzaldehyde |
aniline |
N-(4-bromobenzyl)aniline |
60 |
88 |
|
6 |
4-bromobenzaldehyde |
4-methoxyaniline |
N-(4-bromobenzyl)-4-methoxyaniline |
85 |
90 |
|
7 |
4-methoxybenzaldehyde |
4-bromoaniline |
N-(4-methoxybenzyl)-4-bromoaniline |
100 |
85 |
|
8 |
4-methylbenzaldehyde |
aniline |
N-(4-methylbenzyl)aniline |
100 |
87 |
|
9 |
2-methoxybenzaldehyde |
aniline |
N-(2-methoxybenzyl)aniline |
100 |
85 |
|
10 |
4-methylbenzaldehyde |
4-methoxyaniline |
N-(4-methylbenzyl)-4-methoxyaniline |
90 |
89 |
|
11 |
4-bromobenzaldehyde |
4-bromoaniline |
N-(4-bromobenzyl)-4-bromoaniline |
60 |
90 |
|
12 |
4-methoxybenzaldehyde |
aniline |
N-(4-methoxybenzyl)aniline |
80 |
92 |
|
13 |
4-nitrobenzaldehyde |
4-bromoaniline |
N-(4-nitrobenzyl)-4-bromoaniline |
70 |
90 |
|
14 |
2-methoxybenzaldehyde |
4-methylaniline |
N-(2-methoxybenzyl)-4-methylaniline |
100 |
91 |
Experimental
IR and 1H NMR spectra were recorded on PerkinElmer FT-IR RXI and 400 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples (melting or boiling points). TLC was applied for the purity determination of substrates, products and reaction monitoring over silica gel 60 F254 aluminum sheet.
Reductive amination of banzaldehyde and aniline with NaBH4/NaH2PO4.H2O system (typical procedure)
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer, a solution of benzaldehyde (0.106 g, 1 mmol), aniline (0.093 g, 1 mmol) and NaH2PO4.H2O(0.14, 1 mmol) was preparedin THF (3 mL). Then the NaBH4 (0.036 g, 1 mmol) was added to the reaction mixture and stirred under reflux conditions. TLC monitored the progress of the reaction (eluent; CCl4/Ether: 5/2). The reaction was filtered after completion within 55 min. Evaporation of the solvent and short column chromatography of the resulting crude material over silica gel (eluent; CCl4/Ether: 5/2) afforded the N-benzylaniline (0.l66 g, 92% yield, Table 1, entry 1).
Conclusion
In this context, we have shown that the NaBH4/NaH2PO4.H2O3 as reducing system is efficient for the reductive amination of a variety of aldehydes and anilines to their corresponding secondary amineas. Reduction reactions were carried out with NaBH4 (1 mmol) and NaH2PO4.H2O(1 mmol) in THFunder reflux conditions.Highefficiency of the reduction reactions and easy work-up procedure makes as an attractive new protocol for reductive amination of aldehydes.
Acknowledgements
The authors gratefully appreciated the financial support of this, work by the research council of Islamic Azad University branch of Mahabad.
References
- Alinezhad, H.;Tollabian, Z.Bull. Korean Chem. Soc., 81: 1927( 2010).
- Alinezhad, H.;Tajbakhsh, M.;Mahdavi, N.Synth. Commun.,40: 951( 2010).
- Alinezhad, H.;Tajbakhsh, M.;Hamidi, N.Turk. J. Chem.,34: 307(2010).
- Alinezhad, H.;Tajbakhsh, M.;Zare, M.Synth. Commun.,39: 2907( 2009).
- Heydari, A.; Khaksar, S.;Akbari, J.Esfandyari, M.;Pourayoubi, M.;.Tajbakhsh, M. Tetrahedron Lett.,48:1135( 2007).
- Heydari, A.;Arefi, A.;Esfandyari, M.J. Mol.Catal. A: Chem., 274: 169( 2007).
- Reddy, P. S.;Kanjilal, S.;Sunitha, S.; Prasad, R. B. N.Tetrahedron Lett.,48:, 8807(2007).
- Saidi, M. R.; Stan Brown, R.;Ziyaei-Halimjani, A.J. Iran. Chem. Soc.4:, 194 (2007).
- Cho, B. T.; Kang, S. K.Tetrahedron, 61:5725( 2005).
- Saxena, I.; Borah, R.;Sarma, J. C.J. Chem. Soc., Perkin Trans. 1,503(2000).
- Neidigh, K. A.; Avery, M. A.; .Williamson, J. S.; Bhattacharyya, S.J. Chem. Soc., Perkin Trans. 1, 2527 (1998).
- Varma, R. S.Dahiya, R.Tetrahedron Lett.,54:6293(1998).
- Brussee, J.; van Benthem,R. A.T.M.; Kruse, C.G.; Gen,A. v. d.Tetrahedron: Asymmetry, 1:163(1990).
- Setamdideh, D.; Hasani, S.; Noori, S. J. Chin. Chem. Soc.,60: 1267 (2013).
- Pourhanafi, S.;Setamdideh, D.; Khezri, B. Orient. J. Chem.29: 709 (2013).
- Setamdideh, D.; Karimi, Z.; Rahimi, F. Orient. J. Chem., 27: 1621(2011).
- Setamdideh, D.;Khezri, B.; Mollapour, M. Orient. J. Chem., 27: 991(2011).
- Setamdideh, D.;Khezri, B.;Rahmatollahzadeh, M.;Aliporamjad, A.Asian J. Chem. 24:3591(2012).
- Setamdideh, D.;Rafig, M.E-J. Chem.,9:2345 (2012).
- Setamdideh, D.;Rahmatollahzadeh, M.J. Mex. Chem. Soc.,56:169 (2012).
- Setamdideh, D.;Khezri, B.;Rahmatollahzadeh, M.J. Serb. Chem. Soc.,79:1 (2013).
- Mohamadi, M.; Setamdideh, D.;Khezri, B.Org. Chem. Inter., doi:10.1155/2013/127585 (2013).
- LatifiMmaghani, E.; Setamdideh, D. Orient. J. Chem.29: 953 (2013).
- Kamari, R.;Setamdideh, D.Orient. J. Chem.29: 497 (2013).
- Setamdideh, D.; Khaledi, L. S. Afr. J. Chem., 66: 150 (2013).
- Setamdideh, D.; Karimi, Z.; Alipouramjad, A. J. Chin. Chem. Soc., 60: 590 (2013).

This work is licensed under a Creative Commons Attribution 4.0 International License.









