Recovery of Niobium and Zirconium from the Cyclone Discharge of Chlorination Plant Producing Titanium Tetrachloride
V. S. Gireesh*, V. P. Vinod, S. Krishnan Nair, Georgee Ninan
Research and Development Department, Titanium Pigment Unit, The Kerala Minerals and Metals Limited, Sankaramangalam, Chavara, Kollam, Kerala, India 691583.
DOI : http://dx.doi.org/10.13005/ojc/300132
Article Received on :
Article Accepted on :
Article Published : 01 Feb 2014
This paper describes a method for recovering niobium and zirconium from the cyclone solid residues arising from chlorination of titaniferrous ores. The residue contains beneficiated ilmenite (BI) fines, calcined petroleum coke (CPC) and metal chlorides of niobium, aluminium, zirconium, iron, manganese, vanadium etc. The BI fines and CPC present in the residue were removed by soaking the residue with water and by separating the insoluble fraction contain BI and CPC by filtration. The filtrate containing the soluble metal chlorides was acidified with hydrochloric acid followed by agitation and heating in the presence of sulphate ions (sulphuric acid) to precipitate niobium and zirconium as their oxo sulphate which is filtered, dried and calcined to convert niobium and zirconium oxides. The optimum amount of sulphuric acid was found to be 3 % and the optimum pH and temperature for precipitation of niobium and zirconium is 0.5 and 90 oC respectively.
KEYWORDS:Beneficiated ilmenite; chlorination; Calcined petroleum coke; Niobium pentoxide; Zirconium oxide.
Download this article as:| Copy the following to cite this article: Gireesh V. S, Vinod V. P, Nair S. K, Ninan G. Recovery of Niobium and Zirconium from the Cyclone Discharge of Chlorination Plant Producing Titanium Tetrachloride. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Gireesh V. S, Vinod V. P, Nair S. K, Ninan G. Recovery of Niobium and Zirconium from the Cyclone Discharge of Chlorination Plant Producing Titanium Tetrachloride. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2020 |
Introduction
The chloride route technology for the production of rutile grade TiO2 involves chlorination of the feed stock material mainly beneficiate ilmenite (BI) at high temperature followed by oxidation of the chlorinated product namely TiCl4. The chlorination of titaniferrous material with chlorine in presence of petroleum coke in a fluid bed reactor at a temperature of around 900 oC produce titanium tetra chloride along with chlorides of metal impurities present in the ore. When the chlorination is carried out, a substantial quantity of solids comprise of carbon (calcined petroleum coke), un-reacted ore (BI) and solidified metal chlorides are taken out of the reactor by the effluent gases generated. These solids include significant amount of anhydrous chlorides of vanadium, niobium, titanium, chromium, zirconium, aluminium and iron [1]. In the plant operation these solids are separated from the gaseous stream using cyclone and the solid is quenched with water and pumped to effluent treatment plant (ETP) where it is mixed with lime or caustic and stored in secured ponds. The separation and recovery of the above metals especially niobium and vanadium from the hydrochloric acid solution is very difficult since, recovery process applicable to hydrochloric acid solution remove metals simultaneously [1]. US patent 5,334,362 [2] describe a method for handling the chlorinator waste. The process involved pasting up and filtration of the cyclone dust and the filtrate containing mainly iron (III) chloride is used for waste water treatment. The separation of the un-reacted BI and Calcined petroleum coke (CPC) from the vanadiferrous residue originated by the chlorination of titanium bearing ores by using spirals, forth flotation and wet table methods are reported in the literature [2]. The present study aims at separating and recovering the volatile metal chlorides namely NbCl5 and ZrOCl2 from the residue arising from the chlorination of the titaniferrous ores.
Methods and Materials
Chlorination of BI
The BI, CPC and Chlorine are fed to chlorinator for chlorination at a temperature of 950 oC. At this temperature, the fluidization reaction takes place and all the oxide present in BI were converted to corresponding chlorides. These metal chlorides in the vapour phase, leave the chlorinator bed, along with oxides of carbon, nitrogen, un-reacted BI and CPC. Large portions of the heavy impurity chlorides are separated from the gaseous stream by partial condensation method. They are separated as a free flowing powder along with un-reacted BI and CPC through cyclone. A typical analysis of the waste solid residue is given in Table 1. The carbon content in the cyclone solid was analyzed by using Leco carbon analyzer. The other metal oxides and BI were analyzed by using X-ray Fluorescence (Bruker) and Atomic Absorption Spectrophotometer (Phillips AA 100).
Table 1: Analysis of waste solid residue (all the element present in the residue were expressed as their corresponding oxides)
|
Sl. No. |
Constituents |
Percentage |
|
1 |
Calcined Petroleum Coke |
34.26 |
|
2 |
Beneficiated Ilmenite |
42.60 |
|
3 |
SiO2 |
3.13 |
|
4 |
V2O5 |
1.07 |
|
5 |
Cr2O3 |
0.65 |
|
6 |
Fe2O3 |
13.05 |
|
7 |
Al2O3 |
3.27 |
|
8 |
Nb2O5 |
0.50 |
|
9 |
ZrO2 |
0.10 |
(a) Recovering the un-reacted BI lost through the chlorinator waste
(b) Separation and recovery of metals like Nb, Zr etc from the waste
(c) Effective utilization of the waste, which will otherwise be neutralized and disposed off in the settling ponds, which is a perennial nuisance.
Treatment of cyclone solid
The analysis of the bottom discharge of cyclone revealed that major constituent is petroleum coke and BI. In addition it also contain iron, chromium, zirconium, vanadium,
aluminium and niobium chlorides. They are discharged through the cyclone as fine solids. The solids were collected from the chlorination plant. A known amount of the solid is moistened with water and agitated well. The water addition has to be controlled in order to keep the solution in the highly acidic range, which well prevent the hydrolysis of the chlorides. The solution was allowed to settle overnight and filtered. The constituents present in the residue and filtrate were analyzed by AAS / XRF methods.
Recovery of Niobium and Zirconium
The filtrate collected above was taken for the study of recovering Nb and Zr. Keeping the pH of the solution in the highly acidic range (pH 0.5 to 3), the solution is agitated for 5 hours at 60-90 0C with 98 % H2SO4 (1 to 5 % with respect to the volume of filtrate) as catalyst. During the agitation niobium and zirconium are precipitated out. The solution was filtered, washed and dried. The residue was analyzed for Nb and Zr content by using AAS method.
Results and Discussion
Removal of BI and CPC from cyclone solid
The cyclone solid was moistened with water and agitated overnight. The water insoluble was filtered, dried and de-agglomerated using pestle and mortar. The constituents present in the solid was analyzed and given in Table 2.
Table 2: Analysis data of residue after removing soluble chlorides
|
Sl. No. |
Constituents |
Percentage |
|
1 |
Calcined Petroleum coke |
42.8 |
|
2 |
Beneficiated Ilmenite |
57.2 |
The residue contains 42.8 % of calcined petroleum coke and 57.2 % of beneficiated ilmenite. The separation and re-use of the calcined petroleum coke and beneficiated ilmenite was already reported by the authors [3].
Recovery of Niobium and Zirconium
The filtrate obtained after the removal of BI and CPC was acidified to keep the pH of the solution below 1.0. Then the solution is agitated for 5 hours at 80 0C with 2 % by weight of concentrated H2SO4 as catalyst. During the agitation, niobium and zirconium are precipitated out which was dried and calcined to convert to their corresponding oxides. The precipitated was analyzed to find out the amount of niobium oxide and zirconium oxide present in the residue. The experiment was repeated four times to get the concordant values. The Table 3 gives the amount of niobium oxide and zirconium oxide present in the residue.
Table 3: Analysis data of precipitate (pH – 1.0, temperature- 80 oC, catalyst- 2 %)
| Sl.No. | Constituents |
Percentage |
|||
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | ||
| 1 | Nb2O5 | 45.2 | 49.0 | 48.6 | 45.0 |
| 2 | ZrO2 | 12.0 | 10.8 | 11.5 | 12.1 |
The average composition of Nb2O5 and ZrO2 found in the residue are 47% and11.5% respectively. Table 4 gives the amount of niobium and zirconium separated from the filtrate by varying the pH of the solution and keeping the temperature 80 0C
Table 4: Analysis data of precipitate (pH – varying, temperature – 80 o C, catalyst- 2%)
|
Sl. No. |
pH |
Constituent (%) |
|
|
Nb2O5 |
ZrO2 |
||
|
1 |
3.0 |
30.0 |
8.2 |
|
2 |
2.0 |
36.5 |
8.8 |
|
3 |
1.0 |
48.6 |
11.5 |
|
4 |
0.5 |
50.2 |
12.9 |
As the pH of the solution decreases the amount of niobium and zirconium recovery will increase upto pH 1.0 and below that there was no significant increase. Hence the study was not conducted below the pH of 0.5. Table 5 gives the amount of niobium and zirconium recovered at different temperatures.
Table 5: Amount of niobium and zirconium in the precipitate (pH – 0.5, catalyst- 2%, temperature 60 – 90 oC)
|
Sl. No. |
Temp oC |
Percentage |
|
|
Nb2O5 |
ZrO2 |
||
|
1 |
60 |
35.0 |
8.5 |
|
2 |
70 |
40.2 |
10.0 |
|
3 |
80 |
50.2 |
12.9 |
|
4 |
90 |
50.5 |
12.9 |
As the temperature increases, the amount of niobium and zirconium recovered also increases. The improvement in quantity of recovery above 80 oC was very less and optimum temperature can be fixed as 90 oC. The quantity of niobium pentoxide recovered at the optimum temperature of 90 o C and optimum pH of 0.5 was found to be 50.5% and that of zirconium oxide was 12.9%. The amount of niobium and zirconium precipitated by varying amount of catalyst is given in Figure 1
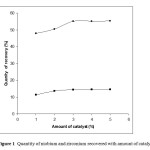 |
Figure 1 Quantity of niobium and zirconium recovered with amount of catalyst Click here to View Figure |
·-Nb2O5 ■- ZrO2, Condition: pH – 0.5, catalyst-1- 5% , temperature -90 o C)
As the catalyst concentration in the medium rises, the recovery of the valuable metal oxides (niobium and zirconium) also increases. A marginal improvement of recovery was noticed above the catalyst concentration of 3% and hence the 3% of catalyst can be considered as the optimum concentration for the reaction. The soluble metal chlorides of niobium and zirconium in the presence of sulphuric acid and at lower pH (~1) form oxo sulphate which precipitate during agitation at elevated temperature (~90 oC). These precipitates were filtered, dried and calcined to obtain the oxides of niobium and zirconium.
Conclusion
A method was developed for recovering niobium and zirconium from residue obtained by the chlorination of titanium bearing ores for producing titanium tetrachloride. The BI fines and CPC in the residue were removed by soaking the residue with water and separated by filtration Acidification of the filtrate obtained after removing the BI and CPC, followed by agitation and heating in presence of sulphuric acid to precipitate a residue containing niobium and vanadium which is then filtered, dried and calcined to converted to corresponding oxides of zirconium and niobium The optimum pH and temperature for the precipitation of niobium and zirconium was found to be 0.5 and 90 oC. The optimum amount of catalyst was found to be 3 %.
Acknowledgement.
The authors are thankfull to Mr C. J. George, Executive Director, KMML, Mr. P. Rajendra Prasad, JGM(T), KMML for their support ,valuable suggestion and giving permission and facility for conducting the study.
References
- Bowerman P. D., US patent 3975495 (1976) “ Metals recovery from Hydrochloric acid solutions”
- Schinkitz D., Thumm H., US patent No 5334362 (1994) “Process for the Production of Disposable Products from Metal Chlorides in Titanium Dioxide Chloride Process”.
- Gireesh V. S., Vinod V. P., Krishnan Nair S., Georgee N., Int. J. Mater, Sci. Innovation, 1(5), 286 (2013) “The recovery of Beneficiated Ilmenite (BI) and Calcined Petroleum Coke (CPC) from the cyclone discharge of chlorination plant producing titanium tetrachloride”

This work is licensed under a Creative Commons Attribution 4.0 International License.









