Reaction 2-R 5-oxo 5-H 6-Ethylcarboxylate 7-Phenyl -1 ,3,4-Thiadiazolo- [3,2-a] Pyrimidine with Amin
Reza Moradivalikboni1*, Zabialah Heidarnezhad2,Fatemeh Heydari2 , Heshmatollahali Nezhad1 and Sahar Mohsenitavakoli1
aDepartment of Chemistry, Mazandaran University, Babolsar, Iran bDepartment of Chemistry,Andimeshk Branch, Islamic Azad University, Andimeshk , Iran
DOI : http://dx.doi.org/10.13005/ojc/300153
Article Received on :
Article Accepted on :
Article Published : 02 Apr 2014
In this article presents Synthesis of 2-R5-oxo 5-H 6 -Carboxamid 7-phenyl 1,3,4-thiadiazolo-[3,2-a] pyrimidine through reaction of 2- R 5 - Oxo 5 - H 6- EthylCarboxilate 7 – phenyl -1, 3,4 – Thiadiazolo-[3,2-a] pyrimidine with amin. The structures of the compounds obtained are set NMR, 13C, IR- spectroscopy.
KEYWORDS:NMR; IR- spectroscopy; 13C; pyrimidine; Thiadiazol
Download this article as:| Copy the following to cite this article: Moradivalikboni R, Heidarnezhad Z, Heydari F, Nezhad H, Mohsenitavakoli S. Reaction 2-R 5-oxo 5-H 6-Ethylcarboxylate 7-Phenyl -1 ,3,4-Thiadiazolo- [3,2-a] Pyrimidine with Amin. Orient J Chem 2014;30(1). |
| Copy the following to cite this URL: Moradivalikboni R, Heidarnezhad Z, Heydari F, Nezhad H, Mohsenitavakoli S. Reaction 2-R 5-oxo 5-H 6-Ethylcarboxylate 7-Phenyl -1 ,3,4-Thiadiazolo- [3,2-a] Pyrimidine with Amin. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2730 |
Introduction
Condensed derivatives of 1,3,4-thiadiazolo[3,2- a]pyrimidinewere reported to possess a broadspectrum of biological activity1-4, including antibacterial,antitumor, fungicidal, and herbicidal properties. However,thiadiazoles and their condensed analogs are still insufficientlystudied. In continuation of the search for substancespossessing increased ability to permeate through biologicalmembranes of various infectious species 5 – 7 and, in particular,for the new antibacterial drugs in these homologousseries of compounds.introduction of a substituent at position 6 of the.The introduction of a substituent at position 6 of the1,3,4-thiadiazolo [3,2-a] pyrimidine system efficientlyenhances the physiological activity of the oleculef8-10.This replacement occurs in the reactions of 1,3,4 -thiadiazolo [3,2-a] pyrimidine derivatives withelectrophiles11-13. In the present work, we studied the possibilities of thesynthesis of various derivatives of 1,3,4-thiadiazolo [3,2 -a] pyrimidine. In Firstwe hav synthesized 2-R5-oxo5-H6-EthylCarboxilate7-phenyl [1,3,4]thiadiazolo[3,2,-a]pyrimidine(3) with Use2- R 5-amino 1,3,4- thiadiazole(1)andethyl 2- formyl 3- okco 3- phenyl propanoate(2). (Figure 1)
![Figure 1.synthesis of 2-R7-phenyl 6- ethylcarboxylate 5-oxo 5-H 1,3,4-thiadiazolo [3,2- a]pyrimidine.](http://www.orientjchem.org/wp-content/uploads/2014/04/Vol30_No1_React_REZA_Fig1-150x150.jpg) |
Figure 1. synthesis of 2-R7-phenyl 6- ethylcarboxylate 5-oxo 5-H 1,3,4-thiadiazolo [3,2- a]pyrimidine. Click here to View Figure |
And more2-R5-oxo5-H6-EthylCarboxilate7-phenyl [1,3,4]thiadiazolo[3,2,-a]pyrimidinereacted with amin(4) until produced 2-R 5-oxo 5-H 6 -Carboxamid 7-phenyl 1,3,4-thiadiazolo-[3,2-a] pyrimidine(5).(Figure 2)
![Figure 2.synthesof 2- R 5-oxo 5-H 6-Carboxamid 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidine.](http://www.orientjchem.org/wp-content/uploads/2014/04/Vol30_No1_React_REZA_Fig2-150x150.jpg) |
Figure 2.synthesof 2- R 5-oxo 5-H 6-Carboxamid 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidine. Click here to View Figure |
In this regard synthes of 2-R 5-oxo 5-H 6-Carboxamid 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidinedo with The aim of 2-R 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1 ,3,4-thiadiazolo [3,2-a] pyrimidine and amin in present solvent C2H5OH.
Result and Discussion
First, we tried synthesisof 2- R 5-oxo 5-H 6-Carboxamid 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidinewith 2-R 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1 ,3,4-thiadiazolo [3,2-a] pyrimidine and amin in variousAlcohol. The reaction performed in the presence ofAlcohol shown in Table 1,
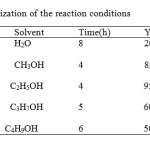 |
Table 1: Optimization of the reaction conditions Click here to View table |
a Yields refer to isolated pure products.
this reaction doesnot take a in the present of catalyst .To show the generality and applicability of this procedure, we treated a wide variety of 2- R 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1 ,3,4- thiadiazolo [3,2-a] pyrimidine and amin in the presence of alcohol ethanol at 78oC and obtained the desirable products in good to excellent yields (Table 2).
![Table 2.synthesisof 2- R 5-oxo 5-H 6-Carboxamid 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidine with 2- R 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1 ,3,4- thiadiazolo [3,2-a] pyrimidine and amina](http://www.orientjchem.org/wp-content/uploads/2014/04/Vol30_No1_React_REZA_T2-150x150.jpg) |
Table 2. synthesisof 2- R 5-oxo 5-H 6-Carboxamid 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidine with 2- R 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1 ,3,4- thiadiazolo [3,2-a] pyrimidine and amina Click here to View table |
Experimental
A mixture of2-R 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1,3,4- thiadiazolo [3,2-a] pyrimidine (1 mmol), amin (1 mmol) was stirred magnetically at 78oC and the progress of the reaction was monitored by thin-layer chromatography (TLC). The reaction mixture was filtered .In all the cases, the product obtained after the usual work up gave satisfactory spectral data. 2-H 5-oxo 5-H 6-Carboxamid 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidine:1H NMR (400 MHz, CDCl3, δ ppm):6 (s, 2H, NH2) ; 7.14-7.30 (5H, Ph)7.50 (S, H).13C NMR (100 MHz, CDCl3, δ ppm):118 (C), 126,4 (CH) , 126,4 (CH) ,128(CH), 128.7(CH), 128.7(CH), 136.9(C),140 (C),162,1(C), 163 (C), 168 (C), 172,6.
Conclusion
In conclusion, the alcohol has been employed as a mild, and highly efficient solvent system for the convenient preparation of 2- R 5-oxo 5-H 6-Carboxamid 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidine in excellent yields from 2- R 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1 ,3,4- thiadiazolo [3,2-a] pyrimidine and amin. In addition low cost, recyclable solvent system. The advantages include low cost, mild reaction conditions and reactions carried out at room temperature with excellent yields.
Acknowledgement
The authors are thankful to the mazandran University Research Council for the partial support of this research and thankful to DrHeshmatollahAlinezhad .
References
- Japan Patent Application No. 63-10794 (1987); Ref. Zh. Khim.,110402P (1989).
- US Patent No. 4742063 (1987); Ref. Zh. Khim., 10401P (1989).
- US Patent No. 4866064 (1989); Ref. Zh. Khim., 70384P (1991).
- Ì. Suiko, S. Hayashida, and S. Nakatsu, Agric. Biol. Chem., 46(11), 2691 – 2695 (1982).
- S. S. Sattorov, M. A. Kukaniev, Z. G. Sanginov, et al., Proc. Rep.Conf.“Advances in Chemistry and Chemical Technology.” [in Russian], Dushanbe (2002), pp. 142 – 144.
- R. A. Gobum and R. A. Glennon, J. Med. Chem., 17(9),1025 – 1027 (1974).
- F. Russo, A. Santagati, M. Santagati, et al., Farmaco. Ed. Sc.,42(6), 437 – 447 (1987).
- M.Suiko and K.Maekawa, Agric.Biol.Chem., 1977,41, 2047.
- M.Suiko, E.Taniguchi, K.Maekawa, and M.Eto, Agric.BioL Chem., 1979, 43, 741.
- M.Suiko, E.Taniguchi, K.Maekawa, and M.Eto, BioLChem., 1979, 43, 747.
- S.Sh.Shukurov, M.A.Kukaniev, I.M.Nasyrov, L.S.Zakharov, and R.A.Kamkhanov, Zh.Obshch.ghim., 1993, ( 63, 2320 [Russ.J.Gen.Chem., 1993, 63(Engl.Transl.)].
- S.Sh.Shukurov, M.A.Kukaniev, 1.M.Nasyrov, L.S.Zakharov, and R.A.Karakhanov, lay.Akad.Nauk, Set.Khim., 1994, 908 [Russ.Chem.BAIL, t994, 43,854(Engl.Transl.)L.
- Y.Suichi, M.Tamotsu, A.Shunzo, F.Hiroyuki, M.Hiroo,M.Mikio, R.Reimei, T.Watan ~, and 1.Sumiro, Chem.AndPharm.Bull., 1992, 40, 3391.

This work is licensed under a Creative Commons Attribution 4.0 International License.









