Extraction of Trace amount Cu(II) by Single Walled Carbon Nanotubes (SWCNTs) in Water Samples and Determination it by FAAS
Hanie Arbabirashid1 And Ali Moghimi1*
1Department of Chemistry, Varamin(Pishva) Branch Islamic Azad University, Varamin, Iran.
DOI : http://dx.doi.org/10.13005/ojc/300124
Article Received on :
Article Accepted on :
Article Published : 14 Mar 2014
A novel, simple method has been developed for the preconcentration of Cu(II) based on the adsorption of its Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) complex on a Single walled carbon nanotubes (SWCNTs) onto C18 cartridge . The influence of acidity, eluting agents, stability of the cartridge, sample volume and interfering ions has been investigated in detail. The adsorbed complex could be eluted using HNO3 4M and the concentration of Cu(II) was determined by flame atomic adsorption spectrometry (FAAS). A detection limit of 0.10 μgL-1 could be achieved and the developed procedure was successfully applied for the determination of Cu(II) in spiked water samples . The preconcentration factor attainable for quantitative recovery (>97%) of Cu(II) was 125 for a 1000mL sample volume. The mentioned method was successfully applied on the determination of Cu(II) in different water samples. In this method, relative standard deviation (RSD) is %1.25.
KEYWORDS:Copper(II); Preconcentration; Solid phase extraction; Single walled carbon nanotubes (SWCNTs). Flame atomic adsorption spectrometry (FAAS).
Download this article as:| Copy the following to cite this article: Arbabirashid H, Moghimi A. Extraction of Trace amount Cu(II) by Single Walled Carbon Nanotubes (SWCNTs) in Water Samples and Determination it by FAAS. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Arbabirashid H, Moghimi A. Extraction of Trace amount Cu(II) by Single Walled Carbon Nanotubes (SWCNTs) in Water Samples and Determination it by FAAS. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2436 |
Introduction
Cu at trace concentrations acts as both a micronutrient and a toxicant in marine and fresh water systems [1-8].This element is needed by plants at only very low levels and is toxic at higher levels. At these levels, Cu can bind to the cell membrane and hinder the transport process through the cell wall. Cu at nearly 40ng mL-1 is required for normal metabolism of many living organisms [9, 10]. On the other hand, Cu is an important element in many industries. Thus, the development of new methods for selective separation, concentration and determination of it in sub-micro levels in different industrial, medicinal and environmental samples is of continuing interest. The determination of Cu is usually carried out by flame and graphite furnace atomic absorption spectrometry (AAS) [11, 12 ]as well as spectrometric methods [13, 14] . Solid phase extraction (SPE) methods are the best alternatives for traditional classic methods due to selective removal of trace amounts of metal ions from their matrices. SPE determinations can be carried out on different efficient ways. One of the most appropriative performation features of SPE is achieved by using octadecyl silica membrane disks. SPE reduce the use of toxic solvent, disposal costs, and extraction time[15-16].The octadecyl silica membrane disks involves shorter sample processing time and decreased plugging due to the large cross-sectional area of the disk and small pressure drop which allows higher flow-rates; reduced channeling resulting from the use of sorbent with smaller particle size and a greater mechanical stability of the sorbent bed[17]. In our previous attempts, we modified SPE membrane disks with suitable compounds for selective determination of chromium [18-19] and lead[22].Meanwhile, other investigators have successfully utilized these sorbents for quantitative extraction and monitoring trace amounts of lead]21,23], copper[24-26], silver[27-28], mercury[29], cadmium[31], palladium[32], Ce[33] and UO230. Ionic liquids (ILs) seem well positioned to address this challenge. Due to their wide solubility, and by introducing a surface charge, modification with ILs should enable the preparation of long-term stable and Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) that can be dispersed in various matrices. To date, investigations into the covalent attachment of an ionic material to Single walled carbon nanotubes (SWCNTs) surface have been not carried out. In this communication, we report a convenient method to obtain polydisperse Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) that are functionalized with 1-(3-aminopropyl) – 3 methylimidazolium bromide (IL-NH2) [20].The main goal of the present work is development of a fast, sensitive and efficient way for enrichment and extraction of trace amounts of Cu(II) from aqueous media by means of a cartridge C18 modified with , Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) [20]. Such a determination has not been reported in the literature. The chelated ions were desorbed and determined by FAAS. The modified solid phase could be used at least 50 times with acceptable reproducibility without any change in the composition of the sorbent, Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) . On the other hand, in terms of economy it is much cheaper than those in the market, like C18 SPE mini-column.
Experimental
Reagents and Apparatus
Single walled carbon nanotubes (SWCNTs) and Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) were prepared from Merck (Darmstadt, Germany). Method and dried for a week over phosphorus pentoxide in a vacuum desiccators before use. 4-Isocyanatobenzenesulfonyl azide was prepared from 4-carboxybenzenesulfonyl azide via a published procedure [17]. All solutions were prepared with doubly distilled deionized water from Merck (Darmstadt, Germany). C18 powder for chromatography with diameter of about 50 µm obtained from Katayama Chemicals from supelco. It was conditioned before use by suspending in 4 M nitric acid for 20 min, and then washed two times with water.
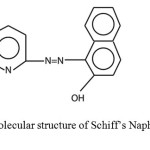 |
Schematic 1 Synthesis and Molecular structure of Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) Click here to View Scheme |
Column preparation
Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) (40 mg) were packed into an SPE mini-column (6.0 cm ×9 mm i.d., polypropylene). A polypropylene frit was placed at each end of the column to prevent loss of the adsorbent. Before use, 0.5 mol L−1 HNO3 and DDW were passed through the column to clean it.
Apparatus
The pH measurements were conducted by an ATC pH meter (EDT instruments, GP 353)calibrated against two standard buffer solutions of pH 4.0 and 9.2. Infrared spectra of Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) were carried out from KBr pellet by a Perkin-Elmer 1430 ratio recording spectrophotometer. Atomic absorption analysis of all the metal ions except Zn(II) were performed with a Perkin-Elmer 2380 flame atomic absorption spectrometer. Zn(II) determinations were performed by a Varian Spect AA-10. Raman spectrophotometer analysis were performed with a Perkin-Elmer. 2.3.1. Preparation of column: to 40 ml of water containing 1.5 g of C18, 150 mg of the above Schiff base-chitosan grafted multiwalled carbon nanotubes was loaded after washing acetone, 4mol l-1 HNO3 solution and water, respectively, solution was added. The pH of the suspension was adjusted to 2.0 by addition of 4 M HNO3 and stirred by mechanical stirrer for 20 min. Then the top liquid was decanted (and discarded) and the remained C18 was washed three times with water, then with 5 ml of 4 M HNO3and again three times with water. The prepared sorbent was transfered to a polypropylen tube (i.d 5 mm, length 10mm). Determination of Cu2+ contents in working samples were carried out by a Varian spectra A.200 model atomic absorption spectrometerequipped with a high intensity hallow cathode lamp(HI-HCl) according to the recommendations of the manufacturers. These characteristics are tabulated in( Table 1). A metrohm 691 pH meter equipped with a combined glass calomel electrode was used for pH measurements.
Table 1 . The operational conditions of flame for determination of Cu
| TaTable 1 . The operational conditions of flame for determination of Cu | |||
| Slit width | 0.7 nm | ||
| Operation current of HI-HCL | 10 mA | ||
| Resonance fine | 283.3 | ||
| Type of background correction | Deuterium lamp | ||
| Type of flame | Air/acetylene | ||
| Air flow | 7.0 mL.min-1 | ||
| Acetylene flow | 1.7 mL.min-1 | ||
Procedure
The pH of a solution containing 100 ng of each Cu(II) was adjusted to 2.0. This solution was passed through the column with a flow rate of 5 ml min-1. The column was washed with 10 ml of water and the retained ions were desorbed with 1 ml of 4 M HNO3 with a flow rate of 2 ml min-1. The desorption procedure was repeated 3 more times. All the acid solutions (4 ml all together) were collected in a 10 ml volumetric flask and diluted to the mark with water. The concentrations of Cu in the solution were determined by FAAS at 283.3.
Determination of Cu in water Samples
Polyethylene bottles, soaked in 1 M HNO3 overnight, and washed two times with water were used for sampling. The water sample was filtered through a 0.45 µm pores filter. The pH of a 1000 ml portion of each sample was adjusted to 2.0(4 M HNO3) and passed through the column under a flow rate of 5 ml min-1. The column was washed with water and the ions were desorbed and determined as the above mentioned procedure.
Speciation of Cu in water samples
This procedure is reported in several articles. The method has been evaluated and optimized for speciation and its application on complex mixtures [26-29]. The chelating cation exchanger(Chelex-100) and anion exchanger, Dowex 1X-8 resins were washed with 1 M HCl, water, 1 M NaOH and water respectively. 1.2 g of each resin was transfered to separate polyethylene columns. Each column was washed with 10 ml of 2 M HNO3 and then 30 ml of water. The C18 bounded silica adsorber in a separate column was conditioned with 5 ml of methanol, then 5 ml of 4 M HNO3and at the end with 20 ml of water. 5 ml of methanol was added on top of the adsorber, and passed through it until the level of methanol reached just the surface of the adsorber. Then water was added on it and connected to the other two columns. A certain volume of water sample was filtered through a 0.45 µm filter and then passed through the three columns system, Dowex 1X-8, RP-C18 silica adsorber and Chelex-100 respectively. The columns were then separated. The anion and cation exchanger columns were washed with 10 ml of 4 M HNO3 and the C18 column with 10 ml of 1 M HCl. The flow rate of eluents was 1 ml min-1. The Cu content of each eluted solution were determined by FAAS.
Results and Discussion
Stability studies
The stability of the Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) phases was performed in different buffer solutions (pH 1, 2, 3, 4, 5, 6 and 0.1M sodium acetate) in order to assess the possible leaching or hydrolysis processes. Because the metal capacity values determined in Section 3.2 revealed that the highest one corresponds to Cu(II)s, this ion was used to evaluate the stability measurements for the Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) phase. The results of this study proved that the Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) is more resistant than the chemically adsorbed analog especially in 1.0, 5.0 and 10.0 M hydrochloric acid with hydrolysis percentage of 2.25, 6.10 and 10.50 for phase, respectively. Thus, these stability studies indicated the suitability of phase for application in various acid solutions especially concentrated hydrochloric acid and extension of the experimental range to very strong acidic media which is not suitable for other normal and selective chelating ion exchangers based on a nano polymeric matrix [9]. Finally, the Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) phases were also found to be stable over a range of 1 year during the course of this work. Primary investigations revealed that cartridge C18 could not retain Cu(II) cations, but when modified with the Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) retains these cations selectively. It was then decided to investigate the capability of the Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) as a ligand for preconcentration and determination of Cu. The cartridge C18 surface in acidic media(1<pH<6) attracts protons and becomes positively charged [22].
Effect of pH in does not occur.
The effect of pH of the aqueous solution on the extraction of 100 ng of each of the cations Cu(II) was studied in the pH rang of 1-10. The pH of the solution was adjusted by means of either 0.01 M H NO3 or 0.01M NaOH. The results indicate that complete chelation and recovery of Cu(II) occurs in pH range of 2-4 and that of in 2-8 and are shown in Fig. 1. It is probable that at higher pH values, the cations might be hydrolysed and complete desorption occur. Hence, in order to prevent hydrolysis of the cations and also keeping on the cartridge C18, pH=3.0 was chosen for further studies.
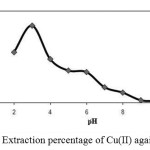 |
Fig. 1. Extraction percentage of Cu(II) against pH. |
Effect of flow rate of the solutions of the cations on chelation of them on the substrate was also studied. It was indicated that flow rates of 1-5 ml min-1would not affect the retention efficiency of the substrate. Higher flow rates cause incomplete chelation of the cations on the sorbent. The similar range of flow rate for chelation of cations on modified cartridge C18 and a Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) has been reported in literature[21,22]. Flow rate of 1-2 ml min-1for desorption of of the cations with 4 ml of 4 M HNO3has been found suitable. Higher flow rates need larger volume of acid. Hence, flow rates of 5 ml min-1and 2 ml min-1were used for sample solution and eluting solvent throughout respectively.
Effect of the Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) quantity
To study optimum quantity of the Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) on quantitative extraction of Cu, 50 ml portions of solutions containing 100 ng of each cation were passed through different columns the sorbent of which were modified with various amounts, between 10-50 mg of the Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs). The best result was obtained on the sorbent which was modified with 40 mg of the Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs).
Figures of merit
The breakthrough volume is of prime importance for solid phase extractions. Hence, the effect of sample volume on the recovery of the cations was studied. 100 ng of each cation was dissolved in 50, 100, 500 and 1000 ml of water. It was indicated that in all the cases, chelation and desorption of the cations were quantitative. It was then concluded that the breakthrough volume could be even more than 1000 ml. Because the sample volume was 1000 ml and the cations were eluted into 10 ml solution, the enrichment factor for both cations are 125, which is easily achievable. The maximum capacity of 1.5 g of the substrate was determined as follow; 500 ml of a solution containing 50 mg of each cation was passed through the column. The chelated ions were eluted and determined by FAAS. The maximum capacity of the sorbent for three individual replecates was found to be 15.2±0.8 µg of each cation. The limit of detection (3σ) for the catoins[30] were found to be 0.10 ng.ml-1for Cu ions. Reproducibility of the method for extraction and determination of 100 ng of each cation in a 50 ml solution was examined. As the results of seven individual replicate measurements indicated, they were 1.25% for Cu(II).
Effect of foreign ions
Effect of foreign ions were also investigated on the measurements of Cu. Here a certain amount of foreign ion was added to 50 ml of sample solution containing 100 ng of each Cu(II) with a pH of 3.0. The amounts of the foreign ions and the percentages of the recovery of Cu are listed in Table 2. As it is seen, it is possible to determine Cu without being affected by the mentioned ions. The prepared sorbent was used for analysis of real samples. To do this, the amounts of Cu were determined in different water samples namely: distilled water, tap water of Tehran (Tehran, taken after 1
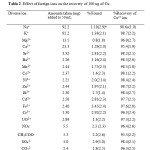 |
Table 2: Effect of Foregin ions on the recovery of 100 ng of Cu. Click here to View table |
0 min operation of the tap), rain water(Tehran, 25 January, 2013), Snow water (Tehran , 7 February ,2013), and two synthetic samples containing different cations. The results are tabulated in Table 3. As it is seen, the amounts of Cu added to the water samples are extracted and determined quantitatively which indicates accuracy and precision of the present method. Separation and speciation of cations by three columns system It is possible to precon- centrate and at the same time separate the neutral metal complexes of Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs), anionic complexes and free ions from each other by this method[27]. Water samples were passed through the three connected columns: anoin exchanger, C18-silica adsorber and chelating cation exchanger.
Analysis of the water samples
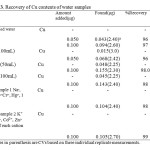 |
Table 3. Recovery of Cu contents of water samples Click here to View table |
Each species of Cu is retained in one of the columns; anionic complexes in the first column, neutral complexes of Schiff’s Naphtol-2-(Pyridylazo-2)-1 (PAN) modified Single walled carbon nanotubes (SWCNTs) in the second, and the free ions in the third. The results of passing certain volumes of different water samples through the columns are listed in Table 4. According to the results, it is indicated that Cu present only as cations. On the other hand the t-test comparing the obtained mean values of the present work with those published indicate no significant difference between them. We have proposed a method for determination and preconcentration of Cu in water samples using cartridge C18 impregnated with a Sciff’s base. The proposed method offers simple, highly sensitive, accurate and selective method for determination of trace amounts of Cu(II) in water samples.
Table 4. Results of speciation of Cu in different samples by three columns system.
|
Table 4. Results of speciation of Cu in different samples by three columns system. |
|||||||
|
River water(50ml)
|
water sample (1000ml)a
|
Tap water (1000ml)
|
|||||
|
Cu(µg)
|
Cu(µg)
|
Cu(µg)
|
Column
|
||||
|
– |
– |
– |
Dowex 1X8
|
||||
|
– |
– |
– |
Silica C-18
|
||||
|
0.103(2.8)
|
0.104(2.9)
|
0.012(4.0)b
|
Chelex-100
|
||||
|
a: This was a solution containing 0.1 µg of each cation in 1000 ml of distilled water. bValues in parenthesis are CVs based on three replicate analysis. The samples are the same as those mentioned in Table 4. |
|||||||
Acknowledgments
The authors wish to thank the Chemistery Department of Shahre Rey branch Islamic Azad University for financial support.
References
- R.M. Izatt, J.S. Bradshaw, S.A. Nielsen, J.D. Lamb, J.J. Christensen, D. Sen, Chem. Rev. 1985,85 ,271.
- R.M. Izatt, K. Pawlak, J.S. Bradshaw, R.L. Bruening, Chem. Rev. 1991,91,1721.
- R.M. Izatt, K. Pawlak, J.S. Bradshaw, R.L. Bruening, Chem. Rev. 1995, 95 ,2529.
- A.J. Blake, F. Demartin, F.A. Deviallonova, A. Garau, F. Isaia, V. Lippolis, M. Schroder, G. Verani, J. Chem. Soc., Dalton Trans. 1996,3705.
- M. Arca, A.J. Blake, J. Casab, F. Demartin, F.A. Devillanova, A. Garau, F. Isaia, V. Lippolis, R. Kivekas, V. Muns, M. Schroder, G. Verani, J. Chem. Soc., Dalton Trans. 2001, 1180.
- V. Ghoulipour, S.W. Husain, Acta Chromatographica, 2002,12, 170.
- O. R .Hashemi,. M. Kargar-Razi,; F.Raoufi, A.Moghimi, H.Aghabozorg, M. R.Ganjali, Microchem. J., 2001,69,1.
- N.I. Shcherbinina, G.V. Myasoedova, T.A. Khabazova, E.Y. Shroeva, G.R. Ishmiyarova, I.E. Nikitina, L.N. Bannykh, Zh. Anal. Khim. 1990, 45 ,2137.
- M.M. Gomes-Gomes, M.M. Hidalgo Garcia, M.A. Palacio Corvillo, Analyst 1995,120,1911.
- Unger K., Porous Silica, Elsevier, Amsterdam, 1979.
- Boudreau S.P., Cooper W.T., Anal. Chem. 1989, 61 ,41.
- Kvitek R.J., Evans J.F., Carr P.W., Anal. Chim. Acta,1982, 144 ,93.
- M.L. Bruening, D.M. Mitchell, J.S. Bradshaw, R.M. Izatt, R.L. Bruening, Anal. Chem. 1991, 63 , 21.
- Mahmoud M.E., Talanta ,1997,45 ,309.
- Mahmoud M.E., Soliman E.M., Talata,1997, 44,15.
- Mahmoud M.E., Soliman E.M., Talanta ,1997,44 ,1063.
- Tong A., Akama Y., Tanaka S., Anal. Chim. Acta ,1990,230 ,179.
- Dadler V., Lindoy L.F., Sallin D., Schlaepfer C.W., Aust. J. Chem. 1987, 40 ,1557.
- H. Ahmad Panahi, M. Abdouss, F. Ghiabi, Elham Moniri,A. Mousavi Shoushtari Journal of Applied Polymer Science impress 2011.
- H. Yang, C.Shan, F. Li, D.Han, Q. Zhang , L. Niu Chem. Commun., 2009, 3880–3882.
- Smith MB, March J. March’s advanced organic chemistry: reactions,mechanisms, and structure. New York: John Wiley & Sons Inc.; 2001. p. 1182–3.
- Moghimi, A. Ghiasi R., Abedin A.R. , Ghammamy S. African Journal of Pure and Applied Chemistry 2009,3 (3), pp. 051-059.
- Harvey Diehl, Clifford C. Hach. Inorg.Synth. Inorganic Syntheses 3: 196–201,(1950).
- Kaiss .R.,Waleed .F and Mohammed A., J.of Al-Anbar university for pure science : Vol.1 : No.1 ( 2007).
- I.V. Mishenina, E.N. Shapovalova, T.A. Bolshova, P.V. Smirnov, O.A. Shpigun, J. Anal. Chem. 51 (1996) 270–276.
- H. Wang, H.S. Zhang, J.K. Cheng, Talanta 48 (1999) 1.
- H.Wang, H.S. Zhang, J.K. Cheng, P.H. Qiu Microchem. J. (1997) 332.
- C.P. Zhang, D.Y. Qi, T.Z. Zhou, Talanta 29(1982) 1119.
- T.Z. Zhou, D.Y. Qi, C.P. Zhang, Acta Chim. Sin. 41(1983) 237.
- Zargaran M., Shoushtari A. M., Abdouss M., J. Appl. Polym. Sci. 2008, 110, 3843.
- P.Nayebi;A. MOGHIMI, Oriental Journal of Chemistry 22(3)(2006)507.
- A. MOGHIMI, Oriental Journal of Chemistry 22(3)(2006)527.
- Dilovic I, Rubcic M, Vrdoljak V, Pavelic S K, Kralj M, Piantanidab I and Cindrica M, Bioorg Med Chem., 2008, 16, 518.

This work is licensed under a Creative Commons Attribution 4.0 International License.









