Comparative Study of Isotherms Adsorption of B12 by Single-wall Carbon Nanotube and Multi-wall Carbon Nanotube
Azin Dehmolaei1, Mehdi Vadi2*
1Department of Chemistry,Gachsaran Branch, Islamic Azad University,Gachsaran,Iran.
2Department of Chemistry,Sarvestan Branch, Islamic AzadUniversity ,Sarvestan, Iran.
DOI : http://dx.doi.org/10.13005/ojc/300133
Article Received on :
Article Accepted on :
Article Published : 27 Jan 2014
This experimental study aimed to compare the adsorption of B12 by twoadsorbent; SWCNTs and MWCNTs by use of Uv-Vis spectrophotometer Jenway 6505model. In this study, four different concentrations of B12 in the range of 212.nm were used. In all conducted experiments, the values of adsorbents, exposure time, temperature, andpH were assumed constant. Based on the results, under similar conditions the efficiency of adsorptionof B12 by MWCNTs was more than SWCNTs. The results can be beneficial inpharmaceutical, oil and construction industries, biology and advanced water and wastewater treatmentplant.
KEYWORDS:Adsorption; Adsorbent; B12; MWCNTs; SWCNTs
Download this article as:| Copy the following to cite this article: Dehmolaei A, Vadi M. Comparative Study of Isotherms Adsorption of B12 by Single-wall Carbon Nanotube and Multi-wall Carbon Nanotube. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Dehmolaei A, Vadi M. Comparative Study of Isotherms Adsorption of B12 by Single-wall Carbon Nanotube and Multi-wall Carbon Nanotube. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=1968 |
Introduction
The first serious attempt to understand thestructure of carbons produced by the pyrolysis oforganic materials was made by Rosalind Franklinin the 1950s 1. She showed that these carbons fallinto two distinct classes, which she calledgraphitizing and non-graphitizing. . Carbon nanotubes are a new form of carbon with unique electrical and mechanical properties2. They can be considered as the result of folding graphite layers into carbon cylinders. Nanotube oxidation of the double tube begins that will open tube. Tubes have high capillarity and can solve liquids and gas in itself3-4. All compounds on the surface of carbon nano-tubes are adsorbed by two major covalent and non covalent links 5-6. They can be planted there without causing ulcers in the point and they can release the drugs slowly over the time in the point. The application part of carbon nano tube are: Precise photography of biological, chemical and biological sensors have a reliable and long life, gene therapy through gene transfer into cells by them, removing bacteria and.….7 Multi-wall nano-tubes discovered in 1991 would create incentives for broader research inengineering science based on entirely carbon nanotubesand their applications8. Multiwalled carbonnanotubes (MWCNTs) can adsorb many atoms and moleculeson their surface such as adsorption of metallic elements like lithium9, potassium10, rubidium11, cesium12 and non-metallic such as hydrogen13, oxygen14, nitrogen15 and methanol16. Adsorption
characteristic of MWCNTs is breather for adsorption of gases such as hydrogen and other gases15. All of the compounds on the surface of MWCNTs adsorbed two main covalent bonds and non-covalent bonds 17-18.This largely due to the favorable combination of properties such havingfaultles structure, a small, low-density, highhardness and strength of the carbon nano-tubes.
Antioxidantsare substancesthatpreventtheformation of free radicalsin cells. Antioxidantsplay an important rolein preventingcancer.Cobalaminor Vitamin B12 is is the protype member of a large family of Antioxidant substances. Designated chemically as α-(5,6-dimethylbenzimidazolyl) cobamid cyanide. It is one of the eight B vitamins. It is normally involved in the metabolism of every cell of the human body, especially affecting DNA synthesis and regulation, but also fatty acid synthesis and energy production19 .
The molecular weight is 1355.37 g/mol. Its molecular formula isC63H88CoN14O14P . Considering the role of vitamins in the diet and its importance in energy metabolism on how to attract them much research has been done with extraordinary properties of carbon nano-tubes as vitamin B12 soluble in water which has particular importance.
EXPERIMENTAL
Chemicals materials
We used the carbon nanotube single wall with 95% and multi wall nanotube with 97% pure degree, production of neutrino Company.Water is distilled twice to prepare vitaminB12 solution.
Methods
At first Solutions used was prepared by solving VitaminB12 and distilled water is used twice. Then, 50ppm of B12 was provided using this sample, some solutions with different concentrations of (8.10.12.14) mg/lit of pure B12 were prepared. Absorbance of four standard solutions was measured by spectrophotometer and calibration curve was plotted. 10 ml of four standard solutions were added separately to 0.005 grams of carbon nanotube single wall and carbon nanotube multi wall as adsorbent and after60 minutes mixing by magnetic mixer solutions. Then liquid and solid phase were separated by means of a filter paper. The concentration of B12 was measured by using on spectrophotometer tool adsorption rate gained for B12. All tests have been performed at the lab with the temperature of (293 ± 1°C).
Adsorption isotherms
The adsorption isotherm described the relationship between the equilibrium concentrations of adsorbate in the solution and the amount of adsorbate on adsorbent. Which indicates how adsorbate molecules are distributed between the liquid phase and solid phase when the adsorption process reaches equilibrium20,21. In this study, three isotherms were used for describing the experimental results, namely the Freundlich isotherm, the Langmuir isotherm and the Temkin isotherm.
Langmuir isotherm
The Langmuir model assumes that theideal monolayer adsorption takes place at specific homogeneous sites within the adsorbent, i.e. once a molecule occupies a sit and no further adsorption takes place 22.The Langmuir equation may be written as
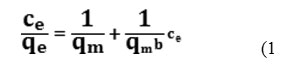
Where qeis the amount of solute adsorbed per unit weight of adsorbent at equilibrium (mg.g-1), Ce the equilibrium concentration of the solute in the bulk solution (mg.L-1).
Freundlich isotherm
The Freundlich isotherm was broadly usedto describe adsorption phenomenon in liquid andfor adsorption on heterogeneous surface with multilayer adsorption. This isotherm assumes that as the adsorbate concentration increases, the concentration of adsorbate on the adsorbent surface also increase23,24. The Freundlich isotherm is expressed by the following empirical equation:
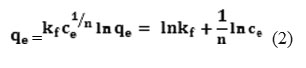
where qe and Ce are the equilibratedconcentration of the adsorbate in sorbent and solution, respectively, where KF is a constant indicative of the relative adsorption capacity of the adsorbent (mg1-(1/n)L 1/ng-1), and n is adsorption intensity related to the surface heterogeneity.
Temkin model
The Temkin isotherm equation assumesthat the heat of adsorption of all the molecules inthe layer decreases linearly with coverage due toadsorbent–adsorbate interactions, and that theadsorption is characterized by a uniform distribution of the binding energies, up to some maximum binding energy25,26. It is expressed by the relation:
qe=B1ln kT+B1lnCe (3)
where constant B=RT/bis related to the heat of adsorption, R the universal gas constant(Jmol-1 K-1), T the temperature(K), b the variation of adsorption energy (J mol-1) and KTis the equilibrium binding constant (Lmg-1) corresponding to the maximum binding energy.
RESULTS AND DISCUSSION
Adsorption isotherms The adsorption data were analyzed accordingto the linear form of the isothermsThe linear plots are shown Fig. 1, 2 and 3.The fitting results, i.e. Isotherm parameters and the coefficient of determination, R2, presented in Table 1. The value of correlation coefficient (293°K)for Freundlich equation (R2 = 0.9994) is higher thanLangmuir (R2= 0.9342) and Temkin (R2 = 0.989)suggesting that equilibrium data arewell describedby Freundlich isotherm
Table 1: Parameters of Langmuir, Freundlich and Temkin isotherms of theVitamin B12 on MWCNTs and SWCNTs
|
|
Langmuir |
|
Freundlich |
|
Temkin |
|
||||||
|
b |
q |
|
n |
|
B |
|||||||
|
MWCNTs |
0.01 |
40.98 |
0.9342 |
0.91 |
2.20 |
0.99 |
2.71 |
4.09 |
0.98 |
|||
|
SWCNTs |
0.16 |
11.9 |
0.7407 |
1.89 |
2.30 |
0.84 |
1.282 |
2.93 |
0.79 |
|||
 |
Fig. 1:Freundlich isotherm of B12 on MWCNT and SWCNT Fig. 3: Langmuir isotherm of B12 on MWCNT and SWCNT Fig. 2:Temkin isotherm of B12on MWCNT and SWCNT
|
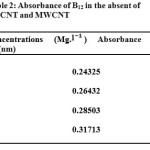 |
Table 2: Absorbance of B12 in the absent of SWCNT and MWCNT Click here to View table |
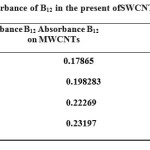 |
Table 3: Absorbance of B12 in the present ofSWCNT and MWCNT Click here to View table |
CONCLUSION
In this study we compare the adsorption isotherms of B12 by carbon nanotube Single and multi wall. Base on obtained results we conclude that MWCNTs has more efficiency in removal of B12 rather than SWCNTs.Therefore, in total, it is concludedthat correlation coefficient, (n and Kf) in Freundlich isotherm model for MWCNT were higher and it’s efficiency in the removal of B12 is better than SWCNT.The results indicate that the Freundlich adsorption isotherm fits the data better than the other two models which suggests heterogeneity in the sorption sites.
References
- R E Franklin. Proc. R. Soc. A 209: 196 (1951).
- PG,CollinsP.Avouris Nanotubes for electronics. Sci.A 2000; 283: 38 – 45.
- J.M.tarascom and M.Rmand, Nature. 395x (2001).
- Jeong-yeolyoon,woo -sikkim, AdsoptiBSA on highly carbboxylate microspheres quantitative effects of surface functional groups and interaction forces, J.Colloid and Interface Sci., 177: 613-620-19
- Bahr,J.L;Tour, J. Mj. Mater. Chem. 2002. 12 (1952)
- Basiuk, E, V:Monroy-Pelaez, Puente-Lee, I:Basiuk,V.A.Nanolett, 4: 863 (2004).
- M. Arulepp, L, Pperma, J.Leis, A.Person, K.Ru,mma, A.Jun (2004).
- S.Ijima, Nature 354: P.56 (1991).
- C.N.R. Rao, B.C. Satishkumar, A. Govindaraj and M. Nath, Phys.Chem.,2, 78 (2001).
- N. Bendiab, E. Anglaret, J.-L.Bantignies, A. Zahab, J.L. Sauvajol, P. Petit, C. Mathis and S. Lefrant, Phys. Rev. B, 64, 245424 (2001)..
- A.S. Claye, N.M. Nemes, A. Janossy and J.E. Fischer, Phys. Rev. B,62, R4845 (2002).
- A.M. Rao, P.C. Eklund, S. Bandow, A. Thess and R.E. Smalley, Nature,3881, 257 (1997).
- A. Wadhawan, R.E. Stallcup and J.M. Perez, Appl. Phys. Lett., 78, 108(2001).
- A. Cao, H. Zhu, X. Zhang, X. Li, D. Ruan, C. Xu, B. Wer, J. Liang and D. Wu, Chem. Phys. Lett., 342, 510 (2001).
- Q.-H. Yang, P.-X.Hou, S. Bai, M.-Z.Wang and H.-M.Cheng, Chem.Phys. Lett., 345, 18 (2001).
- X.Y. Zhu, S.M. Lee and T. Frauenheim, Phys. Rev. Lett., 85, 2757(2000).
- S. Talapatra and A.D. Migone, Phys. Rev. B, 56, 045416 (2002).
- A.C. Dillon and M.J. Heben, Appl. Phys. A, 72, 133 (2001).
- J.M.tarascom and M.Rmand, Nature. 395x (2001).
- Sheng, GD, DD Shao, XM Ren, XQ Wang, JX Li, YX Chen, and XK Wang, Kinetics and thermodynamics of adsorption of ionizable aromatic compounds from aqueous solutions by as-prepared and oxidized multiwalled carbon nanotubes, Journal of hazardousmaterials178: 505-516 (2010).
- Freitas,A.F., M.F. Mendes, and G.L.V. Coelho, Thermodynamic study of fatty acidsadsorption on different adsorbents, J. Chem.Thermodynamics 39: 1027-1037 (2007).
- I. Langmuir, The constitution and fundamental properties of solids and liquids, J. Am. Chem. Soc. 38: 2221–2295 (1916).
- H.M.F. Freundlich, U¨ ber die adsorption in lo¨sungen, Z. Phys. Chem. 57: 385-470 (1906).
- J. Zeldowitsch, Adsorption site energy distribution, ActaPhysicochim. URSS 1: 961- 973 (1934).
- M.I. Temkin, Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and in the interaction between adsorbed molecules, Zh. Fiz. Chim.15: 296– 332 (1941).
- Y. Kim, C. Kim, I. Choi, S. Rengraj, J. Yi, Environ. Sci. Technol. 38: 924 (2004).

This work is licensed under a Creative Commons Attribution 4.0 International License.









