A New Method for Synthesis of 2,4,5-Triaryl-1H-Imidazole Derivatives Using SiO2-NaHSO4 under Solvent-free Conditions
Farhad Hatamjafari and Hadiseh Khojastehkouhi*
Department of Chemistry, Faculty of Science, Islamic Azad University-Tonekabon Branch, Tonekabon, Iran
DOI : http://dx.doi.org/10.13005/ojc/300143
Article Received on :
Article Accepted on :
Article Published : 27 Jan 2014
A mixture of benzyl, aromatic aldehyde and ammonium acetate in presence of SiO2-NaHSO4 under solvent-free condition were converted to 2,4,5-Triaryl-1H-Imidazoles. The short reaction time, cleaner reaction, and easy workup make this protocol practical and economically attractive.
KEYWORDS:Imidazoles; Solvent-free; Multicomponent reactions; SiO2-NaHSO
Download this article as:| Copy the following to cite this article: Hatamjafari F, Khojastehkouhi H. A New Method for Synthesis of 2,4,5-Triaryl-1H-Imidazole Derivatives Using SiO2-NaHSO4 under Solvent-free Conditions. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Hatamjafari F, Khojastehkouhi H. A New Method for Synthesis of 2,4,5-Triaryl-1H-Imidazole Derivatives Using SiO2-NaHSO4 under Solvent-free Conditions. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=1978 |
INTRODUCTION
Multicomponent reactions (MCRs) refers to a reaction in which two or more ingredients are combined within a single process and the products they create, which is part of all the components are present1-3. Since the multi-component reactions for the synthesis of organic compounds and these compounds can be used as a drug and precursor multicomponent reactions, so to investigate them out is important4.
Imidazoles are important heterocycle compounds in medicinal chemistry. Imidazoles widely have been used of biological activity which has made them privileged structures in combinational drug discovery libraries. They have the biological roles and also found application as a chromophore with high extinction coefficient, readily tunable absorption wavelength, and fluorophoric properties and was desirable as a large planar synthetic building block in supramolecular chemistry. Recently, several improved methodologies have been developed that use HY/silica gel, acidic Al2O3, AcOH, ionic liquid, NH4OAc, sodium bisulfate among others5-8. Previously, we have synthesized a number of heterocyclic compounds9-17.
In this study, we have used of SiO2-NaHSO4 as a catalysts to develop a new and easy methodology for the synthesis of 2,4,5-triaryl imidazole derivatives. The experiments were started with the study of one-pot, a simple, mild and efficient method for the preparation of the 2,4,5-triarylimidazoles by using SiO2-NaHSO4 as a catalyst (Scheme1).
Scheme 1
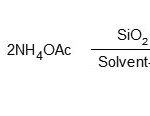 |
Scheme1: Click here to View figure |
EXPERIMENTAL
All chemicals were obtained from Merck or Fluka without further purification. Silica gel SILG/UV 254 plates were used for TLC. IR spectra were measured on a Shimadzu IR-470 Spectrophotometer. 1H NMR spectra were determined on Bruker 500 DRX AVANCE instrument at 500 MHz, respectively.
General procedure for preparation of 2a–l
A mixture of aldehyde (1 mmol), benzil (1 mmol), ammonium acetate (3 mmol) and SiO2-NaHSO4 (10 mol %) as a catalyst was stirred at 120 oC for 30 min. The progress of reaction was monitored by TLC. After finishing, recrystallized from ethanol 95% to give pure products (A1-A4)
spectral data
2,4,5-Triphenyl-1H-imidazole (A1)
Pale yellow crystals, Yield: 0.26 g (89%), mp: 274-276°C. IR (νmax/cm-1)(KBr): 3400(NH Str.); 3000(CHarom Str.); 1600(C=C Str.); 1470(C=N Str.). 1H NMR (400.13 MHz CDCl3)d(ppm): 7.22-7.49(14H, m, CHarom), 7.94(2H,d,J=8Hz,2CH), 9.40(H, s, NH).
2-(4-Chlorophenyl)-4,5-diphenyl-1H-imidazole (A2)
Bright crystals, Yield: 0.28 g (85%), mp: 260-262°C. IR (νmax/cm-1)(KBr): 3400(NH Str.); 3000(CHarom Str.); 1600(C=C Str.); 1500(C=N Str.). 1H NMR (400.13 MHz CDCl3)d(ppm): 7.09-7.84 (11H,m,CHarom),7.82-7.84(2H,d,J=8Hz 2CH), 9.53(H,s, NH).
2-(4-Methoxyphenyl)-4,5-diphenyl-1H-imidazole (A3)
Pale yellow crystals, Yield: 0.28 g (86%), mp: 227-230°C. IR (νmax/cm-1)(KBr): 3442(NH Str.); 3070(CHarom Str.); 1599(C=C Str.); 1450(C=N Str.). 1H NMR (400.13 MHz CDCl3)d(ppm): 3.84(3H,s,CH3);6.93(2H,d,J=8Hz, 2CH); 7.27-7.53(10H,m,10CH),7.82(2H,d,3J=8Hz, 2CH).
2-(3-Nitrophenyl)-4,5-diphenyl-1H-imidazole (A4)
Pale yellow crystals, Yield: 0.3 g (88%), mp> 300°C. IR (νmax/cm-1)(KBr): 3400(NH); 3055(CHarom); 1600(C=C);1475(C=N); 1420,1375(NO2). 1H NMR (400.13 MHz CDCl3)d(ppm): 7.39-7.85(10H, m, CHarom); 7.98-8.0(2H , d, J=8Hz , 2CH); 8.10(1H , s, CH).
RESULTS AND DISCUSSION
We have been able to introduce an efficient and environmentally friendly for the synthesis of imidazole derivatives via condensation of benzil with various aromatic aldehydes and ammonium acetate. Therefore, reported SiO2-NaHSO4 as catalyst which could provide an efficient, cheap, easy separation, high yield and simple route under solvent-free condition for the synthesis of imidazoles.
ACKNOWLEDGEMENTS
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
REFERENCES
- Domling A., Chem. Rev., 106: 17 (2006).
- Khan A. J and Basheer M., Orient. J.Chem., 27(4): 1759-1762 (2011).
- Setamdideh D., Karimi Z and Rahimi F., Orient. J.Chem., 27(4): 1621-1634 (2011).
- Kalinski C., Lemoine H., Schmidt J., Burdack C., Kolb J., Umkehrer M. and Ross G., Syn. lett., 24: 4007 (2008).
- Gadekar L. S., Mane S. R., Katkar S. S., Arbad B. R. and Lande M. K., Cent. Eur. J. Chem., 7: 550 (2009).
- Radziszewski B., Chem. Ber., 15: 1493 (1882).
- Janvier P., Sun X. and Bienayme H., J. Am. Chem. Soc., 124: 2560 (2002).
- Sridhara M. B., Srinivasa G. R. and Gowada D. C., J. Chem. Res. Soc., 1: 74 (2004).
- Azizian J., Hatamjafari F., Karimi A. R. and Shaabanzadeh M., Synthesis. 5: 765 (2006).
- Azizian J., Shaabanzadeh M., Hatamjafari F. and Mohammadizadeh M.R., Arkivoc. (xi): 47 (2006).
- Hatamjafari F., Synthetic Communications. 36: 3563 (2006).
- Azizian J., Hatamjafari F. and Karimi A. R., Journal of Heterocyclic Chemistry. 43: 1349 (2006).
- Hatamjafari F and Montazeri N., Turkish Journal of Chemistry. 33: 797 (2009).
- Hatamjafari F., Orient. J. Chem., 28: 141(2012).
- Hatamjafari F., Orient. J. Chem., 29: 93(2013).
- Hatamjafari F and Alijanichakoli F., Orient. J.Chem., 29: 145(2013).
- Hatamjafari F and Hosseinian A., Orient. J.Chem., 29: 109(2013).

This work is licensed under a Creative Commons Attribution 4.0 International License.









