A Multistep Preparation of 3-Aryl-8-Methoxythiazolo [3’, 2’ : 2, 3] [1, 2, 4] Triazino [5, 6-b] Indoles Under Microwave Ir-radiations
Ravinder Singh
Govt. College for Women, Sampla.
DOI : http://dx.doi.org/10.13005/ojc/300141
Article Received on :
Article Accepted on :
Article Published : 13 Mar 2014
The different 3-Aryl-8-methoxythiazolo [3’, 2’ : 2, 3] [1, 2, 4] triazino [5, 6-b] indoles having antihistaminic, antithyroid, antitubercular, antifungal & antibacterial activities are synthesized through a multistep preparation in high yield in shorter reaction time under microwave irradiations.
KEYWORDS:Indole; Microwave; Aryl; heterocyclic.
Download this article as:| Copy the following to cite this article: Singh R . A Multistep Preparation of 3-Aryl-8-Methoxythiazolo [3’, 2’ : 2, 3] [1, 2, 4] Triazino [5, 6-b] Indoles Under Microwave Ir-radiations. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Singh R . A Multistep Preparation of 3-Aryl-8-Methoxythiazolo [3’, 2’ : 2, 3] [1, 2, 4] Triazino [5, 6-b] Indoles Under Microwave Ir-radiations. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2368 |
Introduction:
Bridgehead nitrogen heterocycles containing thiazole and related heterocycles (thiadiazole and thiadiazine) exhibit antihistaminic, antithyroid, antitubercular, antifungal & antibacterial activities1-3 and their synthetic importance has been greatly enhanced by the recent uses of their condensed bridgehead nitrogen heterocycles as anthelminitics, antidepressants, platelet aggregation inhibitors, antineoplastic, vulcanization accelerators and photographic sensitizers.4-11 The indoles are already been synthesized by different method But they requires longer reaction time and tedious workup.12-21 Microwave assisted reactions are gaining much more importance in synthetic organic chemistry due to dramatic reduction in time from days to hours and hours to minutes or seconds.22-23
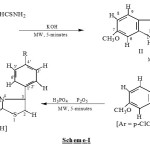 |
Scheme 1: Click here to View Scheme |
The present work reports the synthesis of 3-Aryl-8-methoxythiazolo[3’, 2’ : 2, 3][1, 2, 4] triazino[5, 6-b]indoles in a multi step preparation in high yield in shorter reaction time(Scheme-I). Our work started by reacting 6-methoxyisatin with thiosemicarbazide in Anhyd. ethanol under microwave irradiation at 560W for 5-minutes to give 6-Methoxyisatin-3-thiosemicarbanzone (I). After separation, the 6-Methoxyisatin-3-thiosemicarbanzone(I) reacts with 5% KOH under microwave irradiation at 560W for 5-minutes to give 7-Methoxy-5H-2,3-dihydro [1, 2, 4] triazino [5, 6-b] indole-3-thione (II). The compound 7-Methoxy-5H-2,3-dihydro [1, 2, 4] triazino [5, 6-b] indole-3-thione (II) further reacts with p-chlorophenacyl bromide under microwave irradiation at 560W for 5-minutes to give 5H-3-(p-chlorophenacylthio-7-methoxy [1, 2, 4] triazino [5, 6-b] indole hydrobromide (IIIa; Ar = p-C1C6H4). Similarly, 7-Methoxy-5H-2,3-dihydro [1, 2, 4] triazino [5, 6-b] indole-3-thione(II) was also irradiated with p-cholorophenacyl bromide, phenacyl bromide, under microwave irradiation at 560W for 5-minutes to give 5H-3-(p-bromophenacylthio-7-methoxy [1, 2, 4] triazino [5, 6-b] indole hydrobromide(IIIb, Ar = p-Br C6H4) and 5H-3-(phenacylthio-7-methoxy [1, 2, 4] triazino [5, 6-b] indole hydrobromide (IIIa; Ar = C6H5) respectively. The results are shown in Table-1.
Table:1 3-p-Chlorophenyl-8-methoxythiazolo[3’, 2’ : 2, 3][1, 2, 4] triazino [5, 6-b] indole
|
Sr. NO. |
Substrate(R) |
Time(in minutes) |
Yield(%) |
m.p.(oC) |
|
1. |
-pClC6H4-(IIIa) |
5 |
94 |
> 260OC |
|
2. |
-pBrC6H4-(IIIb) |
5 |
91 |
> 260OC |
|
3. |
-C6H5(IIIc) |
5 |
98 |
> 260OC |
We further explore our work by irradiated 5H-3-(p-chlorophenacylthio-7-methoxy [1, 2, 4] triazino [5, 6-b] indole hydrobromide (IIIa; Ar = p-C1C6H4) in a mixture of H3PO4/P2O5 under microwave irradiation at 560W for 5-minutes to give 3-(p-chlorophenyl-8-methoxythiazolo[3’, 2’ : 2, 3][1, 2, 4] triazino[5, 6-b]indole(IVa, R = Cl). Similarly, 5H-3-(p-bromophenacylthio-7-methoxy [1, 2, 4] triazino [5, 6-b] indole hydrobromide (IIIb; Ar = p-BrC6H4) and 5H-3-(phenacylthio-7-methoxy [1, 2, 4] triazino [5, 6-b] indole hydrobromide (IIIa; Ar = C6H5) were also irradiated in a mixture of H3PO4/P2O5 under microwave irradiation at 560W for 5-minutes to give 3-(p-bromophenyl-8-methoxythiazolo[3’, 2’ : 2, 3][1, 2, 4] triazino[5, 6-b]indole(IVa, R = Br) and 3-(phenyl-8-methoxythiazolo[3’, 2’ : 2, 3][1, 2, 4] triazino[5, 6-b]indole(IVa, R = H) respectively. The results are shown in Table-2.
Table 2: 3-p-Chlorophenyl-8-methoxythiazolo[3’, 2’ : 2, 3][1, 2, 4] triazino [5, 6-b] indole
|
Sr. NO. |
Substrate(R) |
Time(in minutes) |
Yield(%) |
m.p.(oC) |
|
1. |
-Cl(IVa) |
5 |
91 |
> 250OC |
|
2. |
-Br(IVb) |
5 |
90 |
> 250OC |
|
3. |
-H(IVc) |
5 |
96 |
> 250OC |
Experimental
All the melting points reported are uncorrected. Infrared spectra (nmax in cm-1) were recorded in nujol mull or KBr on a Perkin-Elmer 842/Beckman IR-20 / Hitachi 215 spectrometers. The proton magnetic resonance spectra were recorded on a VXR-200 MHz or R-32 Perkin-Elmer 90 MHz spectrometer in CDC13 or DMSO-d6 using tetramethylsilane (TMS) as internal reference stadnard. The chemical shifts are expressed in d (ppm) units downfield from TMS. Mass spectra were scanned on a Jeol JMX-DX-300 spectrometer operating at 70 eV. Carbon, hydrogen and nitrogen analyses were carried out on a Yanaco MT-3 (JAPAN) instrument. Thin layer chromatography (TLC) were performed on silica-gel plates using acetone-benzene (1 : 3 or 1 : 2) as solvent system and iodine chamber as visualizing agent.
Typical procedure for the synthesis of 6-Methoxyisatin-3-thiosemicarbanzone(I)
A mixture of 6-methoxyisatin(0.18g, 0.001 mol) in Anhyd. ethanol (2ml) and thiosemicarbazide (0.1g, 0.0011 mol) in a mixture of water (2 ml) and glacial acetic acid (0.5 ml) was irradiated under microwave irradiation at 560W for 5-minutes. A yellow coloured solid formed during irradiation. The solid was filtered, washed well with water and crystallized from ethanol-DMF furnishing yellow crystals. yield 0.247g (95%), m.p. 265OC.[Found : N, 22.68, S, 12.62. C10H10N4O2S requires N, 22.40; S, 12.80%]; IR : 825, 860 (1, 2, 4-trisubstituted benzene ring), 1115 (C=S), 1125 & 1370 (C-O-C stretching), 1620 (C=N), 1700 (C=O), 3200, 3280, 3400 (NH, NH2).
Typical procedure for the synthesis of 7-Methoxy-5H-2,3-dihydro [1, 2, 4] triazino [5, 6-b] indole-3-thione(II)
6-Methoxyisatin-3-thiosemicarbazone (I, 0.125g, 0.0005 mole) in 5% KOH (3.5 ml) was irradiated under microwave irradiation at 560W for 5-minutes. The reaction mixture was cooled and the insoluble material removed by filtration. The filtrate on neutralisation with dil. HCl gave a yellow solid which was filtered, washed well with water and crystallised from aq. DMF furnishing yellow crystals, yield 0.108 g (92%), m.p. > 260OC [Found : C, 51.91; H, 3.57; N, 23.84; S, 13.58. C10H8N4SO requires C, 51.72; H, 3.45; N, 24.13; S, 13.79%]; IR : 810, 860 (1, 2, 4-Trisubstituted benezene ring), 1150 (C=S), 1170, 1370 (C-O-C stretching), 1590, 1610 (C=N), 3200 (N-H stretching).
Typical procedure for the synthesis of 5H-3-(p-chlorophenacylthio-7-methoxy[1, 2, 4]triazino [5, 6-b] indole hydrobromide (IIIa; Ar = p-C1C6H4)
A mixture of II(0.232 g, 0.001 mol) and p-cholorophenacyl bromide (0.234 g, 0.001 mol) in DMF (6 ml) was irradiated under microwave irradiation at 560W for 5-minutes, and poured into ice-water. The solid thus separated, was filtered, washed with water and crystallized from aq. DMF to give IIIa as yellow crystals, yield 0.415 g (94%), m.p. > 260 [Found: N, 12.20; S, 6.92. C18H14N4O2 SBrCl requires N, 12.03; S, 6.87%]; IR : 810, 860 (1, 2, 4-trisubstituted benzene ring), 1170, 1370 (C-O-C stretching), 1570 (C-N stretching), 1590, 1610 (C=NO, 1690 (C=O), 3180 (N-H stretching). Following members of the series were also prepared in a similar way : IIIb (Ar = p-BrC6H4-), yield (91%), m.p. > 260OC [Found : N, 11.23; S, 6.38, C18H14N4O2SBr2 requires, N, 10.98; S, 6.27%]; IR: 1570 (C-N stretching), 1610 (C=N), 1690 (C=O), 3190 (N-H). IIIc (Ar = C6H5), yield (98%) m.p. > 260OC [Found : N, 14.62; S, 8.18.C18H15N4O2SC1 requires N, 14.49; S, 8.27%]; IR : 1575 (C-N stretching), 1600 (C=N), 3240 (N-H stretching).
Typical procedure for the synthesis of 3-p-Chlorophenyl-8-methoxythiazolo[3’, 2’ : 2, 3][1, 2, 4] triazino [5, 6-b] indole(IVa, R = C1)
Ketone IIIa(0.1g) in a mixture of H3PO4 (0.3ml) and P2O5 (0.4g) was irradiated under microwave irradiation at 560W for 5-minutes. The reaction mixture was poured into water and neutralised with aq. K2CO3 solution. The solid, thus separated, was filtered, washed well with water and crystallised from aq. DMF to furnish IVa as dark red crystals, yield 0.071 g (91%), m.p. > 2500 [Found : C, 59.17; H, 3.11; N, 15.16; S, 8.91.C18H11N4SOCI requires C, 58.93; H, 3.01; N,15.27; S, 8.73%]; IR; 1515 (C-N stretching) 1600 (C=N). PMR (DMSO-d6) : 3.95 (3H, s, C8-OCH3), 7.40 [2H, d (J=7.5Hz), H-3’ and H-5’], 7.70 [2H,d (J = 7.5.Hz), H-2’ and H-6’], 7.90 (1H,s,C2-H), 6.9-8.1 (3H, m, aromatic protons of indole moiety). Following members of the series were also prepared in a similar way: IVb (R = Br): yield (90%), m.p. > 250OC [Found : C, 52.38; H, 2.76; N, 13.42; S, 7.51.C18H11N4SOBr requires C, 52.55; H, 2.67; N, 13.62; S, 7.78%]; IR : 1520 (C-N stretching), 1610 (C = N). IVc (R = H) : yield (96%), m.p. > 250OC[Found : C, 64.92; H, 3.68; N, 16.64; S, 9.87.C18H11N4SO requires C, 65.06; H, 3.61; N, 16.86; S, 9.63%]; IR : 1520 (C-N stretching), 1610 (C = N).
Acknowledgment
We thank Professor D. Villemin (France), Dr. R. Sharma (Dayton, USA) and Professor A.J. Bellamy (Swindon, UK) for inspiration.
References
- Jag Mohan, G.S.R. Anjaneyulu & Kiran, Indian J. Chem. 27B, 128(1988).
- H.R. Snyder, Jr. and L.E. Benjamin, J. Med. Chem., 9, 402 (1966).
- Norwich Pharmacal Co. Neth. Appl. 6,400,380 (1964); Chem. Abstr., 62,2780(1965).
- D.L. Trepanier and P.E. Krieger, U.S. Pat., 3,641,019 (1972); Chem. Abstr., 76, 127024k (1972).
- C.J. Sharpe, R.S. Shadbolt, A. Ashford and J.W. Ross, J. Med. Chem., 14, 977 (1971).
- M.M. KOchhar and B.B. Williams, J. Med. Chem., 15, 322 (1972).
- S. Kano and T. Noguchi, Japan Pat., 71 37,836 (1971); Chem. Abstr., 76, 25295g(1972).
- E.E. Renfrew and H.W. Pons, U.S. Pat., 3,950,130 (1976); Chem. Abstr., 85, 22753e (1976).
- P.W. Jenkins and L.G.S. Brooker, U.S. Pat., 3,681,081 (1972); Chem. Abstr., 78, 85949z (1973).
- M. Hinata, K. SHiba, H. Takei, A. Sato and T. Sakai, Ger. Offen., 2,418,278 (1974); Chem. Abstr., 82,49815K (1975).
- L.G.S. Brooker, U.S. Pat., 2,089,729 (1937); Chem. Abstr. 31, 6989 (1937).
- (a) Romagnoli, R; Baraldi, P.G; Cruz-Lopez, C Preti, D Bermejo, J Estavez, F. Chem. Med. Chem. 2009, 4, 1668. (b) Bursavich, M. G Gilbert, A.M Lombardi, S Georgiadis, K. E; Reifenberg, E4 Flannery, C. R; Morris, E.A. bioorg. Med. Chem. Lett. 2007,17, 5630. (c) Konkel, M.J Packiarajan, M Chem. H Topiwala, U.P4 Jimenez, H Talisman, I.J Coate, H Walker, M.W. Bioorg. Med. Chem. Lett, 2006, 16, 3950. (d) Lam, P.Y.S Vinoent, G Clark, C.G Dcudon, S Jadhav, P.K. Tetrahedron Lett, 2001, 42, 3415.
- Shindikar, A.V Khan, F Viswanathan, C.L. Eur.J.Med. Chem. 2006,41, 786. (b) Moser, P4 Sallmann, A Wieserberg, I.J. Med. Chem. 1990, 33, 2358. (c) Sarges, R Howard, H. R Koe. H.K Weissman, A.J.Med. Chem. 1989, 32, 437.
- Peet, N.J. Heterocycl, Chem. 1990, 17, 1514.
- For reviews, see: (a) Chem, Y Larock, R.C. In Modern Arylation Methods, Ackerman, J. ED Wiley/ VCH New York, 2009, PP401. (b) Sanz, R.Org. Prep. Proced. Int. 2008, 40, 215.
- Lin, Z; Larock, R. C. J. Org. Chem. 2006, 71, 3198. (b) Lin, Z; Larock, R. C. Org. Lett. 2003, 5, 4673. (c) Lin, Z; Larock, R. C. Org. Lett. 2004,6, 99.Lin, Z; Larock, R. C. J. AR.Chem. Sec. 2005, 127, 13112.
- Lin, Z; Larock, R. C. J. AR.Chem. Sec. 2005, 127, 13112.
- Pintori, D. G Greaney, M.F.Org. Lett. 2010, 12, 168.
- Yoshida, H Shirakawa, E4 Honda, Y Hiyama, T. Angew. Chem, Inted. 2002, 41, 3246.
- Zhao, J Larock, R.C.J.Org. Chem. 2007, 72, 583.
- Rogness D. C Larock, R.C. Tetrahedron Lett 2009, 50, 4003.
- Lin, Z; Larock, R.C.J. Org. Chem. 2006, 71, 3198.
- Peddibhotla, S. Curr. Bioact. Compd. 2009, 5, 20.

This work is licensed under a Creative Commons Attribution 4.0 International License.









