2(3H)Pyrrolone – a Biologically Active Scaffold (A Review)
Yakub Ali1, Mohammad Sarwar Alam1*, Hinna Hamid1, Asif Hussain2
1Department of Chemistry, Faculty of Science, Jamia Hamdard, NewDelhi-110062, India. 2Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Jamia Hamdard, New Delhi-5rt110062, India.
DOI : http://dx.doi.org/10.13005/ojc/300101
Article Received on : January 25, 2014
Article Accepted on : March 06, 2014
Article Published : 18 Mar 2014
Pyrrolones are potent medicinal scaffolds and exhibit a broad spectrum of biological activities. This review throws light on the detailed synthetic approaches which have been used for the synthesis of pyrrolones. This has been followed by an in depth analysis of the pyrrolones with respect to their medicinal significance. This review may help the medicinal chemists to develop new leads possessing pyrrolone nucleus with higher efficacy.
KEYWORDS:Pyrrolones; Benzyl Pyrrolone; anti-cancer; anti-inflammatory; anti-microbial activity.
Download this article as:| Copy the following to cite this article: Ali Y, Alama S.M, Hamida H, Hussain A. 2(3H)Pyrrolone – a Biologically Active Scaffol. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Ali Y, Alama S.M, Hamida H, Hussain A. 2(3H)Pyrrolone – a Biologically Active Scaffol. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2502 |
Introduction
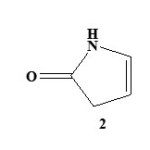
Pyrrolones are the five-membered heterocyclic lactams which are either Δ3 (1) or Δ4 (2) derivatives described by several authors1,2.
 |
Scheme 1 Click here to View Scheme |
These are also known as pyrrolin-2-ones. The γ-lactone ring present in butenolide (3) derivatives possess significant reactivity and has been utilized in the synthesis of pyyrolone derivatives with potent biological acitivity3,4. Pyrrolone and N-benzyl pyrrolone derivatives were reported to have good antifungal, antibacterial and anti-inflammatory activities5. Pyrrolones have been proved important as chiral synthons used in the preparation of a variety of bioactive compounds6. The frequent use of these compounds as chiral intermediates provides a fast universal method for the determination of absolute configuration.7 The chemistry of these versatile building blocks has been explored by a number of groups. Because of their multifunctional nature, these heterocycles can take part in several stereo selective transformations like conjugate additions 8,9, cycloadditions10,11, acyliminium ion chemistry12 and allylic substitution13.
Cycloadditions
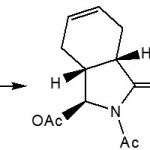 |
Scheme 2: Cycloadditions Click here to View figure |
Conjugate additions
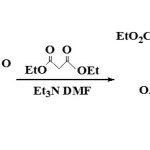 |
Conjugate additions Click here to View figure |
Acyliminium ion
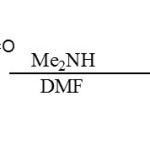 |
Acyliminium ion Click here to View figure |
The interest concerning these pyrrolinones is in part due to the presence of pyrrolic systems, which are also present in the natural products14 viz vitamin B1215, prodigiosin16, bile-pigments17 and some antibiotics like distamycin.18
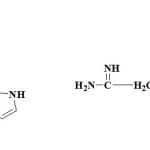 |
Figure Click here to View figure |
Synthetic methodology of pyrrolinones:
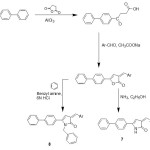
Synthesis of pyrrolones & N-benzylpyrrolones from 3-arylidene-5-biphenyl-2(3H) furanones :
 |
Synthesis of pyrrolones & N-benzylpyrrolones from 3-arylidene-5-biphenyl-2(3H) furanones Click here to View figure |
3-Arylidene-5-biphenyl-2(3H) pyrrolones (7) have been prepared by treating 3-arylidene-5-biphenyl-2(3H) furanones with dry ammonia gas in absolute ethanol. 3-Arylidene-5-biphenyl-1-benzyl-2(3H) pyrrolones (8) have been reported to be synthesized by reacting appropriate furanones with benzylamine in dry benzene to give γ-ketobenzylamides which on lacatamization in 6N HCl give N-benzyl-pyrrolones5.
 |
Synthesis of 1-substituted-5-aryl-4-aroyl-3-hydroxy-3-pyrrolin-2-ones: From ester of pyruvic acid derivatives Click here to View figure |
Synthesis of 1-substituted-5-aryl-4-aroyl-3-hydroxy-3-pyrrolin-2-ones:
From ester of pyruvic acid derivatives
The reaction between equimolar amounts of esters of pyruvic acid with a mixture of aromatic aldehydes and amines gives 1-substituted-5-aryl-4-aroyl-3-hydroxy-3-pyrrolin-2-ones (9) 7, 19,-21.
 |
Figure Click here to View figure |
Instead of ester of pyruvic acid derivatives, esters of 4-aryl-2,4-dioxobutanoic acids22 with aliphatic amines like 2-(N,N-diethylamino)ethylamine and aldehydes in ethanol at room temperature gives good yield.
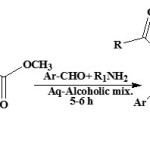 |
Figure Click here to View figure |
Reactions conducted in aqueous alcohol mixtures with short time heating gives good yield7.
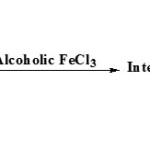 |
Figure Click here to View figure |
Pyrrolinones so obtained appear as light-yellow crystalline substances insoluble in water and soluble in ethanol, chloroform and DMSO.20-22All these compounds develop a characteristic intense cherry-red colour on reacting with an alcoholic solution of iron chloride37.
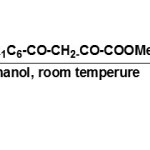 |
Figure Click here to View figure |
The interaction of benzoylpyruvic acid with mixture of aromatic aldehydes and amines involves several competing reactions. For this reason, the products include both substituted 4-benzoyl-3-hydroxy-2,5-dihydro-2-pyrrolones, benzoylpyruvic acid amides.23 1-(4-Antipyryl)-5-aryl-4-aroyl-3-hydroxy-3-pyrrolin-2-ones (16) have been synthesized by reacting esters of 4-aryl-2,4-dioxobutanoic acids with Schiff bases (15), which are obtained by the condensation of aromatic aldehydes with 4-amino antipyrine22. To a solution of 0.001 mole of a methyl ester of 4-aryl-2,4-dioxobutanoic acid in ethanol, a mixture of ethanolic solutions of benzaldehyde (0.001 mole) and 4-amino antipyrine (0.001 mole) was added at room temperature. The excess of solvent is distilled off and the product is crystallized from ethyl acetate22.
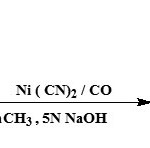 |
Figure Click here to View figure |
Synthesis of unsaturated hydroxy-butyrolactones under phase transfer conditions
Unsaturated hydroxy-butyrolactones or 2-alkylidene-3-ketocarbocyclic acids (17) can be synthesized by carbonylation of α-ketoalkynes in the presence of Ni(CN)2 under phase-transfer conditions24.
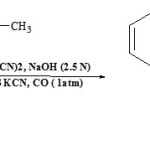 |
Figure Click here to View figure |
By changing the reaction conditions it is possible to obtain hydroxyl-butyrolactams (18) in good yields25.
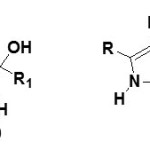 |
Figure Click here to View figure |
However the formation of hydroxylactams by hydrocyanation of α-ketoalkynes in water under mild conditions, it is possible to obtain hydroxyl butyrolactams (19) and (20)26
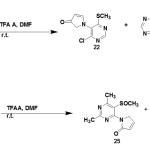 |
Figure Click here to View figure |
Synthesis of Δ3 and Δ4-pyrrolinones by oxidation of pyrrole:
Using sulfoxide electrophillic sulfenylation:
Δ3 Pyrrolinones with very small amounts of Δ4 isomer can be obtained by the oxidation of pyrrole25 during sulfoxide electrophilic sulfenylation (SES). Sulfoxide electrophilic sulfenylation (SES) is a useful technique for the synthesis of heterocycles. Pyrrole-containing sulfoxides have been exploited for intramolecular sulfenylation to form a wide variety of N,S-heterocycles.
 |
Figure Click here to View figure |
Typical (SES) reaction conditions via two possible reaction pathways accounts for pyrrolinones formation during SES cyclization.27-29 Upon treatment with trifluro acetic acid (TFAA) (DMF, room temperature) compound (21) produced the expected SES product only as minor product(23) and the major product was the pyrrolinones (22). Similarly compound (24) produced a mixture of pyrrolinones (25) and (26) SES cyclization product30-32.
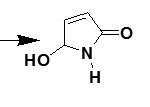 |
Figure Click here to View figure |
Synthesis of an enantioselective enzymatic transesterification:
N-Methyl derivatives of Δ3-pyrrolinones have been synthesized from pyrroles using an enantioselective enzymatic transesterification33. N-Methyl pyrrolinone (28) have been synthesized by photo-oxidation of N-methylpyrrole (27) followed by esterification. Subsequent enzymatic resolution by Candida antarctica mediated transesterification has been reported.
 |
Figure Click here to View figure |
Synthesis of N-acyl derivatives of Δ3-pyrrolinones from methoxy furanones:
N-Acyl derivatives of Δ3-pyrrolinones (30) have been reported to be synthesized from 5-methoxy-3-furan-2-one (29) using an enantioselective enzymatic transesterification.33
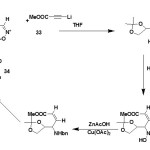 |
Figure Click here to View figure |
Synthesis of C-5 substituted –δ3-pyrrolin-2-ones:
From nitrone:
C-5 substituted-δ3-pyyrollinones (31)have been synthesized from nitrones17.
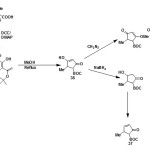 |
Figure Click here to View figure |
The addition of lithium propiolate (33) to nitrone (32) takes place with complete syn selectivity and in quantitative yield to afford the prop-2-ynyl hydroxylamine.34,35
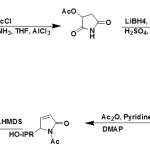 |
Figure Click here to View figure |
The selective hydrogenation of the triple bond was achieved in 1 hr at ambient temperature and 1 atm pressure using the lindlar catalyst. The corresponding allylhydroxylamine was obtained in quantitative yields36. Further deoxygenation of hydroxylamine was accomplished with Zn-Cu catalyst in acetic acid as a solvent. Under these conditions, resulting allylamine could not be isolated since it cyclized spontaneously to the pyrrolin-2-one (34) which was obtained in 84% yield after purification by column chromatography37. This is a straight forward method of preparing enantiomerically pure δ3-pyrrolin-2-ones which can serve as templates for the construction of a number of highly functionalized 2-pyrrolidones and pyrrolidines. The latter has attracted much attention due to their ability to act as selective glycosidase inhibitors with a variety of therapeutic effects.38-40
From N-boc protected amino acids:
N-boc protected 5-methyl-δ3-pyrrolinone (37) has been synthesized from N-boc protected amino acids (35) by Jouin41 and further improved by Ma42. (5S)-N-tert-butoxycarbonyl-4-hydroxy-5-methyl-3-pyrroli-2-one (36) was synthesized from N-boc protected L-alanine and meldrum’s acid which was then reduced with NaBH442 and hydroxyl group of resulting compound was eliminated to get N-boc protected 5-methyl-pyrrolinone43,44.
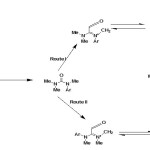 |
Figure Click here to View figure |
From (S)-malic acid:
5-Isopropoxy-δ3-pyrrolin-2-ones (39) can be obtained via stereo-selective synthetic route starting from (S)-malic acid (38)43,45.
 |
Figure Click here to View figure |
Synthesis of isomeric pyrrolones by flash vacuum pyrrolysis of bis aminomethylene derivatives of meldrum’s acid:
Under flash vacuum pyrrolysis46,47 (FVP) conditions (600°C, 0.005 Torr) bis-amino methylene derivatives of meldrum’s acid (40) were transformed into mixtures of the pyrrolones (41). FVP provides a simple and direct synthetic route to 1-substituted-3-hydroxypyrroles and their tautomers i.e. 1H-pyrrol-3-(2H)-ones. Its mechanism was investigated by Brown and Eastwood57 and more recently by Wentrum et al.48.
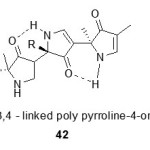 |
Figure Click here to View figure |
Spectral Properties:
Circular dichroism (CD) spectra:
In case of the simple 3-pyrrolin-2-ones bearing no substituents on the olefinic bond, the maximum of the π-π* cotton effect is observed at λmax 200 nm. The maximum of the π-π * cotton effect is seen at longer wavelength, around 230 nm, where the UV spectrum displays a broad shoulder. The 3-pyrrolin-2-ones with an oxygen substituent at C (5) generally display much stronger cotton effects than those containing alkyl group. A methoxy substituent at C(3) shifts the positions of the π-π * band to λmax 230 nm, as does tosyl substitution at N1. 3-Pyrrolin-2-ones with an imide-type group (R2 = acyl) display an additional cotton effect at 280 nm, presumably due to a second π-π * transition. Such double π-π * cotton effects are observed at 250 nm in a saturated imides43 and result from the transitions involving combinations of the carbonyl n orbitals of opposite symmetry. This cotton effect however is much smaller than the n-π* cotton effects caused primarily by the α,β-unsaturated lactam chromophore49-52.
Spectral data of representative compounds:
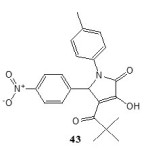 |
Figure Click here to View figure |
1H NMR: In structure A, the chemical shift (δ) value at 6.44-6.95 ppm confirmed the presence of proton at C-4 of pyrrolone ring. Also, the chemical shift ranging between 7.39- 7.68 showed the presence of olefinic proton at C-3 position. Both these peaks appeared as singlet which further evidenced that these are not aromatic protons. The proton attached to N of pyrrolone ring generally appeared at δ = 7.92-8.10. The formation of benzyl pyrrolones (structure B) could be confirmed by disappearance of -NH proton and appearance of a sharp singlet at δ = 4.74-4.88. (methylene proton). IR: The IR spectra of structure A showed the absorption bands at 1680-1710 cm-1 due to the stretching vibrations of the lactam carbonyl groups21. MS: Generally, the mass spectra 3(H)-pyyrolin-2-ones showed peaks at M+ and M+– Ar with high intensities26.
Biological Action:
Various types of pharmacological activities shown by pyrrolinones are as follows:
Antimicrobial activity:
A series of 5-aryl-4-acyl-1-(N,N-dimethylaminoethyl)-3-hydroxy-3-pyrrolin-2ones were found to show weak bacteriostatic activity55. Also various compounds of type 1-substituted-5-aryl-4-aroyl-3-hydroxy-3-pyrrolin-2-ones and derivatives were found to be bacteriostatic against Staphylococcus aureus and Escherichia coli39. 3-Arylidene-4-(biphenyl)- 2(3H)-pyrrolones 5, 3-Arylidene-5-(substituted)- 2(3H)-pyrrolones 56, 3-Arylidene-5-(4-phenoxy phenyl)-2(3H)-pyrrolones57 and 3-Arylidene-5-(4-Methyl)- 2(3H)-pyrrolones58were found to have good antimicrobial activity.
List of compounds showing anti microbial activity
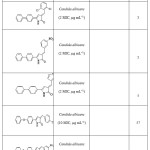 |
List of compounds showing anti microbial activity Click here to View figure |
Antiviral activity:
A number of synthesized pyrrolinones has shown antiviral activity. N-methylated-3,5-linked bis-pyrrolin-4-ones derivatives have been found to be orally bioavailable inhibitors of the HIV-1 protease59-62.
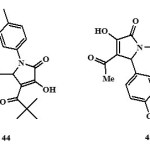 |
Figure Click here to View figure |
Antitumour activity:
The compound 3-hydroxy-5-nitrophenyl-4-pivaroyl-2,5-dihydro-2-pyrrolone (43) exhibits antitumour activity19and inhibited the growth of lung cancer (Tumour Growth = 45%) and CNS cancer (Tumour Growth = 33%)19.
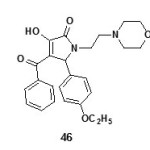 |
Figure Click here to View figure |
Nootropic / Antiamnesic activity:
The compounds 3-hydroxy-5-nitro phenyl-4-pivaroyl-2,5-dihydro-2-pyrrolones (44)19 and 4-Acetyl-5-(3,4-dimethoxy-phenyl)-3-hydroxy-1-1(2-piperidin-1-ylethyl)-2,5-dihydro-2-pyrrolones (45)63 were found to have antiamnesic activity. The drugs were characterized by the ability to eliminate amnesia, increase the latent period and change the number of animals in the test group visiting a dark compartment of the experimental box. The time of stay in the light and dark compartments was determined three minutes after drug injection19. All synthesized compounds favored recovery of the memory trace in the test rats, as evidence by a drop in the number of animals visiting the dark compartment and the time of stay there64.
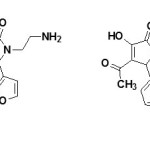 |
Figure Click here to View figure |
Anti-inflammatory activity:
2-Arylidene-4-biphenyl-but-3-en-4-olides and their corresponding pyrrolones and N-benzyl-pyrrolones4 (Table 1), 2-Arylidene-4-(4-phenoxy-phenyl) but-3-en-4-olides and their corresponding pyrrolones and N-benzyl-pyrrolones57 (Table 2) were found to exhibit anti-inflammatory activity. A series of 3-Arylidene-5-(substituted aryl)-1-benzyl-2(3H)-pyrrolones were also found to have promising anti-inflammatory activity56 (Table 3). But the anti-inflammatory activity decreased by the substitution of oxygen atom of butenolide ring with NH (pyrrolones), while substitution of oxygen atom with benzylamine moiety (N-benzyl-pyrrolones) markedly increased the activity. A series of 3-arylidene-5-(4-chloro-phenyl)-2(3H)-benzyl pyrrolones were found to show promising anti-inflammatory activity65. (Table4).
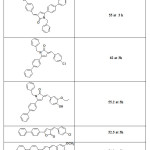 |
Table 1. Anti-inflammatory activity of 2-Arylidene-4-biphenyl-but-3-en-4-olides and their corresponding pyrrolones and N-benzyl-pyrrolones. Click here to View table |
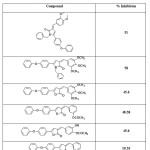 |
Table 2. Anti-inflammatory activity of 2-Arylidene-4-(4-phenoxy-phenyl)but-3-en-4-olides and their corresponding pyrrolones and N-benzyl-pyrrolones. Click here to View table |
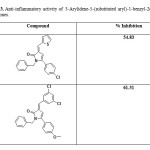 |
Table 3. Anti-inflammatory activity of 3-Arylidene-5-(substituted aryl)-1-benzyl-2(3H)-pyrrolones. Click here to View table |
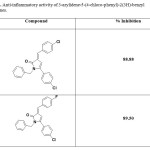 |
Table 4. Anti-inflammatory activity of 3-arylidene-5-(4-chloro-phenyl)-2(3H)-benzyl pyrrolones. Click here to View table |
Analgesic activity:
A series of 3-Arylidene-4-(4-phenoxy-phenyl)1-benzyl-2-(3H) pyrrolones 57 and 3-Arylidene-4-(4-chloro -phenyl)1-benzyl-2-(3H) pyrrolones65 were found to have promising analgesic activity. The compounds of type 3-hydroxy-1,5-diaryl-4-pivaloyl-2,5-dihydro-2-pyrrolones and their derivatives were found to have potent analgesic activity63.
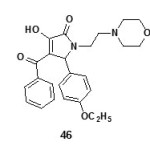 |
Figure Click here to View figure |
Some compounds showing good analgesic activity are shown in Table 5.
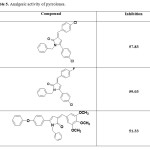 |
Table 5. Analgesic activity of pyrrolones. Click here to View table |
A series of disubstituted-1-(2-amino ethyl)- 3-hydroxy-3-pyrroline-2-ones and their derivatives have been reported to posses showed analgesic activity.66
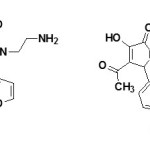 |
Figure Click here to View figure |
Conclusion
It may be concluded that pyrrolones and N benzyl pyrrolones exhibit a broad spectrum of biological activities like anti-inflammatory, anticancer, antibacterial, antifungal, antiviral, analalegic and anti-HIV activities. Thus the pyrrolone scaffold still remains as therapeutic target for the development of new leads in the modern medicinal chemistry. The physical, chemical and the pharmacokinetic properties of pyrrolones still maintains the importance of this moiety inspite of the rising levels of drug resistance in today’s era.
References
- Langenbeck, W.; Boser, H., Chem. Ber., 84, 526 (1951): Grab, C. A.; Anklihelv, P., Chim. Acta., 32, 2010 (1949).
- Bordner, J.; Rpoport, H., J. Org. Chem., 30, 3824 (1965): Atkinson, J. H.; Johnson, A. W., J. Chem. Soc., 5999 (1965).
- Awad, W. I.; Hashem, A. I.; El-Badry, K., Ind. J. Chem., 13, 1139 (1975).
- Khattab, S. A.; Honsy, M., Ind. J. Chem., 19B, 1038 (1980).
- Khan, M. S. Y.; Husain, A., Pharmazie, 57(7), 448-452 (2002).
- Casiraghi, G.; Spanu, P., Rassu, G ; PINNA, L.; Ulgheri, F. J. Org. Chem., 59, 2906 (1994).
- Gawronski, J. K.; Oeveran van, A.; Deen Van Deer, H.; Leung, C. W.; Feringa, B. L., J. Org. Chem., 61, 1513 (1996).
- Jimenez, M. D.; Ortega, R.; Tito, A.; Farina F., Heterocycles, 27, 73 (1988).
- Koot, W. J.; Hiemstra, H.; Speckamp, W. N., Tetrahedron:Asymmetry, 4, 1941 (1993).
- Koot, W. J.; Hiemstra, H.; Speckamp, W. N., J. Org. Chem., 57, 1059 (1992).
- Cooper, D. M.; Grigg, R.; Hargreaves, S.; et al., Tetrahedron, 51, 7769 (1995).
- Koot, W. J.; Hiemstra, H.; Speckamp, W. N., Tetrahedron Lett., 3, 7969 (1992).
- Cuiper, A. D.; Kellog, R. M.; Feringa, B.L., Chem. Commun., 655 (1998).
- Birchall, G. R.; Hughes, C. G.; Rees, A. H., Tetrahedron Lett., 4879 (1970).
- Bonnet, J. R.; Canon, J. R.; Clark, A. W.; Johnson, A. W.; et al., J. Chem. Soc., 1158 (1957).
- Raport, H; Holden, K. G., J. Am. Chem. Soc., 84, 635 (1962).
- Salmon, M.; Diaz, E.; Rock, M. C.; Fensalu, C., Org. Magn. Reson., 126 (1976).
- Grehn, L.; Ragnarsson, U., J. Org. Chem., 46, 3492 (1981).
- Koz’minykh, V. O.; Lgidov, N. M.; Zykova, S. S.; et al., Pharma. Chem. J., 36(4), 188-191 (2002).
- Gein, V. L.; Kasimova, N. N.; Voronina, E. V.; Gein, L. F., Pharma. Chem. J., 35(3), 151-154 (2001).
- Gein, V. L.; Gein, L. F.; Porseva, N. Yu.; Voronina, E. V.; et al., Pharma. Chem. J., 32(9), 477 (1998).
- Gein, V. L.; Pitirimova, S. G.; Voronina, E. V.; et al., Pharma. Chem. J., 31(11), 603 (1997).
- Bezmaternykh, E. N., Author’s abstract of Cand. Sci. Chem. Thesis [in Russia], Perm (2000).
- Arzoumanian, H.; Magali, J.; Didier, N.; Cabrera, A.; Garcia, J. L.; Rosas, N., Organometallics, 14, 5438 (1995).
- Arzoumanian, H.; Magali, J.; Didier, N.; Cabrera, A.; Garcia, J. L.; Rosas, N., Organometallics, in press.
- Rosas, N.; Chavez, M. I.; Garcia, J. L.; Sharma, P.; et al., Analytical Sci., 14, 585-588, june (1998).
- Xia Shao; Kohrt, J.; Dallas, K. Bates, Dept. of Chem., Michigan Tech. Univ., Houghton, MI, 49931, ECHET96 Article 005: Masaru Tada.
- Gilchrist, T. L.; John Wiley and Sons, Heterocyclic Chemistry, 2nd edition , New York, 207 (1992).
- Epstein, W. W.; Sweat, F. W., Chem. Rev., 67, 247 (1967).
- Yamanaka, H.; Abe, H.; Sakamoto, T., Chem. Pharm. Bull., 25, 3334 (1977).
- Bates, D. K.; Habib, Q. A., J. Heterocycl. Chem., 32, 1447 (1995): Bates, D. K.; Tafel, K. A., J. Org. Chem., 59, 8076 (1994): Tafel. K. A.; Bates, D. K., J. Org. Chem., 57, 3676 (1992).
- Russell, G. A.; Mikol, G., J. Mechanisms of Molecular Migrations; Thyagarajan, B. S. Ed.; Interscience Publishers: New York, 1, 157-207 (1968).
- Deen van der, H.; Cuiper, A. D.; Hof, R. P.; et al., J. Am. Chem. Soc., 118, 3801 (1996).
- Merino, P.; Anono, S.; Castilloo, E.; Merchan, F.; Tajero, T., Tetrahedron:Asymmetry, 7 (1996).
- Merchan, F.; Merino, P.; Tajero, T., Theochem., 34, 321(1996).
- Merchan, F.; Merino, P.; Tajero, T.,Departmento de Quimica, ICMA, Universidad de Zaragoza, CSIC, Zaragoza, Spain.
- Winchester, B.; Fleet, G. W., J. Glycobiol., 2, 199 (1992).
- Fleet, G. W. J., Top. Med. Chem., 65, 149 (1988): Legler, G., Adv. Carbohydr. Chem. Biochem., 48, 319 (1990).
- Casiraghi, G.; Zanardi, F.; Rassu, G.; Spanu, P., Chem. Rev., 95, 1677 (1995).
- Spanu, P.; Rassu, G.; Ulgheri, F.; Zanardi, F.; et al., Tetrahedron Lett., 52, 4829 (1996): Poli, G.; Cioli, F.; Maccagni, E.; et al., Tetrahedron Lett., 36, 8669 (1995).
- Jouin, P.; Castro, B., J. Chem. Soc., Perkin Trans., 1, 1177 (1987).
- Ma, D.; Ma, J.; Ding, W.; Dai, L., Tetrahedron:Asymmetry, 7, 2365 (1996).
- Decico, C. P.; Grover, P., J. Org. Chem., 61, 3534 (1996).
- Mattern, R. H., Tetrahedron Lett., 37, 291 (1996).
- Hopman, J. C. P.; Hiemstra, H.; Speckamp, W. N., Chem. Communi., 617 (1995).
- Mcnab, H.; Jones, R. A.; Monahan, L, C., in Pyrroles Part-2; New York, 525 (1992).
- Mcnab, H.; Gaber, A. M, Synthesis, 2059 (2001).
- Wentrup, C.; Rao, V. V. R.; Frank, W.; et al., J. Org. Chem., 64, 3608 (1999).
- Rassu, G.; Casiraghi, G.; Spanu, P.; pinna, l.; Fava, G. G.; et al., Tetrahedron:Asymmetry, 3, 1035 (1992).
- Nikitin, K. V.; Andyukhova, N. P., Mendeleev Commun., 4, 168-170 (1999); C.A., 131(18), 243131w (1999).
- Kateva, A. V.; Gein, L. F.; Gein, V. L.; Aliev, Z. G., Russ. J. Gen. Chem., 69(4), 668-669 (1999): C.A., 131(24), 322498z (1999).
- Liu, X.; Zang, L.; Van der Schyf, C. J.; et al., Chem. Res. Toxicol., 12(6), 508-512 (1999): C.A., 131(7), 84260u (1999).
- Gein, V. L.; Shumilovskikh, E. V.; Andreichikov, Yu. S.; et al., Khim.- Farm. Zh., 30(12), 37-40 (1996).
- Gein, V. L.; Popov, A. V.; Kolla, V. E.; et al., Khim.- Farm. Zh., 27(5), 42 (1993).
- Gein, V. L., Kasimova, N.N., Voronina, E.V.Gein., L.F, Pharm.; Chem., 5 31-34 (2001).
- Khan, M. S. Y.; Husain, A., Sharma., S., Ind. J. Chem., 41(B), 2160-2171 (2002).
- Husain, A., Khan, M. S., A., Hasan, S.M., Alam, M. M., Eur. J. Med, 40(12), 1394-404 (2005).
- Husain, A., Hasan, S.M., L., Sukhibir., Alam, M. M., Ind. J. Chem., 68(4),536-538 (2006).
- Smith, A. B.; Favor, D. A.; Sprengeler, P. A.; et al., Bioorg. Med. Chem., 7(1), 9-22, jan (1999).
- Polonski, t.; Milewska, M. J.; Gdaniee, M., J. Org. Chem., 58, 3134 (1993).
- Koot, W. J.; Hiemstra, H.; Speckamp, W. N., Chem. Communi., 156 (1993).
- Dondoni, A.; Franco, S.; Junquera, F.; Merchan, F.; Merino, P.; Tajero, T., Synth. Communi., 24, 2537 (1994).
- Gein, V. L., Yushkow, V.V.; Kasimova, N.N., Shuklina, M. S.; Vasilenia., M. Yu., Pharm.; Chem., 39 33-39 (2005).
- Casiraghi, G.; Zanardi, F.; Rassu, G.; Spanu, P., Chem. Rev., 95, 1677 (1995).
- Alam, M. M., Hussain, A., Hasan, S. M., Suruchi, Anwer, T., Eur. J. Med.44(6), 2636-2642, ( 2009).
- Gein, V. L., Yushkow, V.V.; Kasimova, N.N., Shuklina, M. S.; Vasilenia., M. Yu., Pharm.; Chem., 39 484-487 (2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.









