Studies on the Analysis of Lead and Silica in Lead Processing Samples
R. Ravichandra Babu1*, Y.V.S. Saikrishna 2
1Department of chemistry, Gitam Institute of science, Gitam University, Visakhapatnam, AndhraPradesh, India 2Research scholar, Department of chemistry, Gitam University, Visakhapatnam. Andhra Pradesh, India
DOI : http://dx.doi.org/10.13005/ojc/290461
Article Received on :
Article Accepted on :
Article Published : 15 Jan 2014
In Practice two samples have to be proceeded separately to determine both lead and silica in lead process samples like concentrates, coarse sinter etc. In the present recommended procedure, the lead and silica are precipitated together by using acid fuming, but, instead of using ammonium acetate treatment to separate lead and silica, a mixture of hydrochloric acid-sodium chloride is used to achieve the same purpose. This helps in determine all the components of lead process samples from the same solution without loss of accuracy in any of the components. This method saves considerable time and especially useful for routine analysis in process control lab.
KEYWORDS:Lead;Silica;Blast furnace Slag;Sinter Coarse;Fluxing compounds
Download this article as:| Copy the following to cite this article: Babu R. R, Saikrishna Y.V.S. Studies on the Analysis of Lead and Silica in Lead Processing Samples. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Babu R. R, Saikrishna Y.V.S. Studies on the Analysis of Lead and Silica in Lead Processing Samples. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1945 |
Introduction
Silica, along with lime and iron oxide, is an important fluxing component in the metallurgy of lead by the conventional blast furnace route 1. The hardness and structure of lead sinter is influenced as much by silica as by lime and iron oxide 2 .The composition of the fluxing components such as silica, iron oxide lime and alumina are to be maintained properly to obtain a good free flowing slag at the temperatures of operation so as to have productivity of standard lead. Silica from hard coke added in the blast furnace is also taken into account while fixing the fluxing components. Further lead is another important element to be analyzed in lead blast furnace slag to monitor the losses of lead along with silica.
Though the analysis3-7 of lead processing samples is practiced since long in smelter industries and procedures are recorded in literature, certain interesting observations 8, 9 have been made with the behavior of silica in these procedures and consequent introduction of errors. If lead has to be determined from the filtrate after silica determination, the whole filtrate has to be evaporated and fumed with sulphuric acid to precipitate lead as lead sulphate. This becomes a very tedious procedure. Further if the initial digestion is conducted using sulphuric acid fuming to precipitate silica and lead together ,the subsequent ammonium acetate treatment to solubulise lead will also lead to some losses of silica9 , thereby making silica determination from the residue a little inaccurate. The other fluxing components can however be determined form the solution after separating both lead and silica
The present study deals with the studies and develops a procedure for the satisfactory and accurate analysis of lead processing samples for the determination of lead and silica simultaneously for all its components which could be more useful for a process control laboratory
Experimental
Reagents
All the chemicals used are of analytical grade
Concentrated hydrochloric acid, nitric acid, sulphuric acid, hydrofluoric acid, perchloric acid, hexamine powder and ascorbic acid are from BDH Analar grade.
Xylenol orange indicator: 200 mg of xylenol orange tetra sodium salt is dissolved in 100ml water.
Hydrochloric acid-sodium chloride mixture: Diluting 200ml of concentrated hydrochloric acid with 1500ml of water and saturating with sodium chloride.
Ammonium acetate solution (20%W/V): 200gm of ammonium acetate are dissolved in 1 lit of water.
Procedure
About 0.5-1.0 gm sample is weighed into a 250ml beaker and 5 ml of water is added and shaken thoroughly so as to make the sample wet.10-15 ml of concentrated hydrochloric acid is added gradually from a measuring jar, the beaker covered with a watch glass and the contents of the beaker are heated gently for 15mts.5ml of concentrated nitric acid is added and heated to a syrupy state 5 ml of hydrochloric acid is added further ,the digestion repeated to expel nitrous oxide fumes and contents are heated to dryness. The beaker is removed from the source of heat, cooled and 5 ml of sulphuric acid is added slowly from the sides of the beaker .The mixture is heated until dense fumes are evolved. The beaker is removed from the hot plate, cooled, 50ml distilled water is added and the contents of the beaker mixed with a glass rod. The solution is boiled gently on an asbestos pad for a few minutes and the beaker is cooled in water. The contents are transferred slowly through Whatman 1 filter paper .The precipitate is washed with 2%V/V sulphuric acid and later with several portions of water, until it is free from acid. The filtrate is diluted to a known volume, normally ml and analyzed for other components like lime, zinc oxide, iron oxide, copper etc., by standard procedure. The residue is proceeded ass follows for the determination of silica and lead
Silica
The residue is transferred into the same beaker and ml of hydrochloric acid-sodium chloride salt solution is added. The contents are allowed to boil for 10-15mts on a pad to avoid bumping. The contents are filtered through ash less pulp or whatman No.40 filter paper .The residue is washed several times with hot water and the entire residue transferred to a funnel The filter paper or the pulp with the residue is dried and ignited in a weighed platinum crucible in a muffle furnace at a temperature of 850-9000c for 1hr.The crucible is removed from the furnace ,cooled in a desecator and weight of the crucible with the residue is noted. The residue is then treated with 4 drops of 1:1 sulphuric acid an 10-15 ml of hydrochloric acid and heated on a hot plate until it gets dried. Later the crucible is ignited in a muffle furnace and silica determined from the loss in weight.
Lead
The filtrate is diluted to a volume of 500 ml and 30-50 mg ascorbic acid and 6-10 drops of xylenol orange indicator added stirring after each addition. The PH of the solution is adjusted to about 6.0 by gradually adding 1gm portions of hexamine with stirring .Addition of hexamine is stopped when the solution turns to reddish purple colour. The solution is then titrated with EDTA .At the end point the colour changes to lemon yellow.
Results and Discussion
The recommended procedure was tested for lead concentrates from various sources like coarse sinter, blast furnace slag. The results obtained for lead and silica from the same sample are compared with the values obtained for silica and lead taking separate samples using standard procedures. The results obtained are presented in Table 1.Since the purpose of present work was also to analyze the other routine components such as lime, iron oxide, zinc oxide ,alumina from the same portion of the sample, the fluxing components are also analyzed from the filtrate solution after removal of lead and silica. The results obtained are presented in Table 2 and Table-3.These results also are compared with the values obtained with the existing practices, wherein two separate samples are processed to achieve the same.
t1 t2 t3
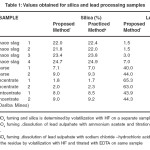 |
Table 1: Values obtained for silica and lead processing samples Click here to View table |
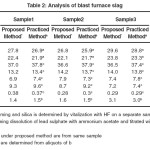 |
Table2: Analysis of blast furnace slag Click here to View table |
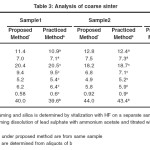 |
Table3: Analysis of coarse sinter Click here to View table |
Conclusion:
The procedure now developed by the author enables the determination of lead, silica without loss of accuracy in lead processing samples .In addition all other fluxing components can also be determined in accuracy from the solution of the same sample .This method saves considerable time and labour as the analyst need not resort to use of two separate samples .This method especially very useful for process control laboratories of lead manufacturing samples.
Acknowledgements: The author is thankful to the management of M/S Hindustan Zinc Limited, Visakhapatnam for providing samples and necessary facilities to carry out the study.
References:
- Young R.S., Chemical Analysis in Extractive Metallurgy, Griffin, London, 188-302(1971).
- Furman N.H., Standard Methods of Chemical Analysis, 6th edn. Vol.1, Krieger, Huntington, 955-957(1975).
- Hillenbrand W.J, Lundell G.E.F., Bright H.A and Hoffman J.I., Applied Inorganic Analysis, 2 nd Edn. Wiley, New York, pp 674-680(1955).
- Agarwal B.C. and Jain S.P., A Text Book of Metallurgical Analysis, Khanna, Delhi, 67 & 84(1980).
- Chalmers R.A in Taylor, H.F.W.D. Editor, Chemistry of Cements, Academic Press, New –York, P. 171(1964).
- Hey M.H., Mineralogy, Mag., 39, 4(1973).
- Bennett H., Analyst. 102,153(1977).
- Rajan S.C.S., Vijaya Ramayya S., Srinivas N.B.V. and Sharma B.H.K., Paper presented at the Annual Convention of Chemists (Indian Chemical Society), Shivaji University, Kolhapur, India (1987).
- Murti S.S. and Rajan S.C.S, Anal.Chim.Acta. 219,177(1989).

This work is licensed under a Creative Commons Attribution 4.0 International License.









