One-pot Synthesis of Quinazolinone and Benzamide Derivatives Using SBA-Pr-SO3H as a Nanoporous Heterogeneous Acid Catalyst
Monireh Shakiba Nahad, Ghodsi Mohammadi Ziarani*
Department of Chemistry; Alzahra University; Vanak Square; Tehran; Iran
DOI : http://dx.doi.org/10.13005/ojc/290443
Article Received on :
Article Accepted on :
Article Published : 27 Dec 2013
A series of quinazolinone and benzamide derivatives have been efficiently synthesized in good to excellent yields via the reaction of 2-aminobenzamide and aromatic benzoyl chlorides under solvent-free conditions using SBA-Pr-SO3H as a nano solid acid catalyst.
KEYWORDS:Quinazolinones; benzamide; SBA-Pr-SO3H; nano-reactor; green synthesis.
Download this article as:| Copy the following to cite this article: Nahad M. S, Ziarani G. M. One-pot Synthesis of Quinazolinone and Benzamide Derivatives Using SBA-Pr-SO3H as a Nanoporous Heterogeneous Acid Catalyst. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Nahad M. S, Ziarani G. M. One-pot Synthesis of Quinazolinone and Benzamide Derivatives Using SBA-Pr-SO3H as a Nanoporous Heterogeneous Acid Catalyst. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1243 |
INTRODUCTION
Quinazolines are an important class of heterocycles with a wide range of pharmacological and biological activities such as anticancer, antiviral, anti-tubercular agents, treatment of diabetes and obesity, anti-inflammatory, insecticidal, anti-microbial1, anticonvulsant, antiulcer, hypolipidemic activity, potent chemotherapeutic agents in the treatment of tuberculosis2, potent and selective ALK5 inhibitors3. These compounds also have sedative, tranquilizer, analgesic, anesthetic, antihypertensive, diuretic, and muscle relaxant properties4-5.
Quinazolinone moiety is a building block for approximately 150 naturally occurring alkaloids6. Luotonin A, Bouchardatine1, and Febrifugine as naturally occurring alkaloids have quinazolinone skeleton. Methaqualone, the most well-known synthetic quinazolinone drug synthesized for the first time in 1951, has sedative–hypnotic effects (Fig. 1)7.
In the most common approach for the synthesis of quinazolinone compounds, 2-aminobenzoic acid or its derivatives, 2-aminobenzamide, 2-aminobenzonitrile, isatoic anhydride, 2-carbomethoxyphenylisocyanate, N-arylnitrilium salts, and 4H-3,1-benzoxazinones as appropriate precursors were used. Other methods involve the reaction of anthranilic acid and the appropriately substituted imidate in a facile one-pot procedure, cycloaddition of anthranilic acid iminoketene to methylbutyrolactam (via sulfonamide anhydride), reactions of anthranilic acid derivatives with a wide range of substrates including imidates and imino halides, and microwave-promoted reaction of anthranilic acid with amines and formic acid (or its ortho ester) and isatoic anhydride2, 7-8.
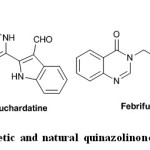 |
Fig. 1: Synthetic and natural quinazolinone compounds Click here to View figure |
Various catalysts such as pentamethylcycloopentadienyliridium(III) chloride dimer ([Cp*IrCl2]2 (Cp*=pentamethylcyclopentadienyl)9, montmorillonite K-1010, I211, InCl31, Ga(OTf)312, [Et3NH]3PW12O4013, carbon based-solid acid5, and tetrabutylammonium bromide (TBAB)7 were used in the synthesis of quinazolinone derivatives.
In continuation of our previous studies14-16, in this paper we want to report the application of SBA-Pr-SO3H as a highly active nanoporous heterogeneous acid catalyst in the preparation of quinazolinone derivatives. The heterogeneous catalysts can suitably be removed from the reaction mixture, making the experimental procedure simple and eco friendly. SBA-Pr-SO3H has mesoporous silica structure with porous texture which is uniform in size (2–10 nm range) that can act as a nano-reactor in organic synthesis17.
Experimental
Materials and Methods
All chemicals were obtained commercially and used without further purification. GC-Mass analysis was performed on a GC-Mass model: 5973 network mass selective detector, GC 6890 Agilent. IR spectra were obtained with a Brucker 500 scientific spectrometer. The 1H NMR was run on a Bruker DPX, 250 MHz. Melting points were measured using the capillary tube method with an electro thermal 9200 apparatus. SEM analysis was performed on a Philips XL-30 field-emission scanning electron microscope operated at 16 kV, while TEM was carried out on a Tecnai G2 F30 at 300 kV.
SBA-15 Nanoporous Silica Synthesis and Functionalization
The nanoporous compound SBA-15 was synthesized and functionalized according to our previous report18-19 and the modified SBA-15-Pr-SO3H was used as nanoporous solid acid catalyst in the following reaction.
General Procedure for the Synthesis of Quinazolinone Derivatives (4a-e)
The SBA-Pr-SO3H (0.01 g) was activated in vacuum at 100 °C and then after cooling to room temperature, 2-aminobenzamide (3 mmol, 0.41 g) and benzoyl chlorides (3 mmol) were added to it. The mixture was stirred at 130 °C under solvent free conditions as reported time in Table 3. After completion of reaction that was indicated by TLC (n-hexane/EtOAc, 1/1), the crude product was dissolved in hot convenient crystallization solvent (Table 3), the heterogeneous solid catalyst was removed easily by simple filtration and after evaporation of excess solvent, corresponding products were obtained. The acid catalyst can be reactivated by simple washing subsequently with diluted acid solution, water and acetone, dried under vacuum and re-used for several times without loss of significant activity.
RESULTS AND DISCUSSION
In this paper, condensation of 2-aminobenzamide 1 with benzoyl chlorides 2 in the presence of SBA-Pr-SO3H as a heterogeneous and reusable nano catalyst for the synthesis of benzamide derivatives 3 and some quinazolinone derivatives 4 was studied (Scheme 1).
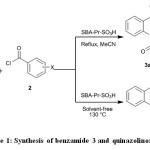 |
Scheme 1: Synthesis of benzamide 3 and quinazolinones 4 Click here to View figure |
At first, the reaction of 2-aminobenzamide 1 and 3,5-dinitro benzoyl chloride 2 was examined as a model experiment in various solvents such as polar protic (H2O, EtOH) and polar aprotic (MeCN, EtOAc, THF) solvents under reflux condition to get an insight into the solvent effect on the yield of reaction. As shown results in Table 1, it can be seen that yield of products 3 were increased in polar aprotic solvents (Table 1, entries 1-4) while in polar protic solvents, the product was formed in slightly lower yield (Table 1, entries 5-6). As shown results in Table 1, among the tested solvents, the best result was obtained in MeCN under reflux conditions in excellent yield. Therefore, MeCN under reflux condition was selected as optimized condition and developed to synthesis other derivatives (Table 2, Method A).
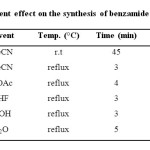 |
Table 1: Solvent effect on the synthesis of benzamide derivatives 3 Click here to View table |
GC-MSS analysis of condensation products of 2-aminobenzamide 1 with various benzoyl chlorides 2 in the presence of SBA-Pr-SO3H under reflux conditions in MeCN as solvent (Method A), indicated that this optimum condition was not applicable for all benzoyl chlorides. The effect of temperature in this reaction was also studied by carrying out the same model of reaction under solvent-free conditions (Method B). As shown in Table 2, the progress of the reaction for the synthesis of quinazolinone derivatives 4 at 130 °C is greater. Therefore, it was observed that the yield of products is a function of temperature and the yield increased as the reaction temperature was raised.
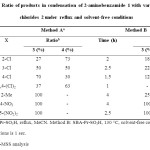 |
Table 2: Ratio of products in condensation of 2-aminobenzamide 1 with various benzoyl chlorides 2 under reflux and solvent-free conditions Click here to View table |
In this regard, different substituted quinazolinones 4a-e were synthesized under solvent-free conditions at 130 °C (Table 3). Results in the synthesis of various substituted benzamides 3a-h are summarized in Table 4.
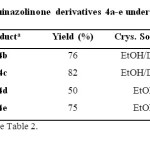 |
Table 3: Synthesis of quinazolinone derivatives 4a-e under solvent-free conditions Click here to View table |
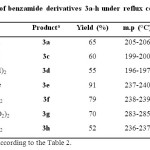 |
Table 4: Synthesis of benzamide derivatives 3a-h under reflux conditions in MeCN Click here to View table |
Suggested mechanism for the synthesis of benzamides 3 and quinazolinones 4 was shown in Scheme 2. SBA-Pr-SO3H as a nano acid catalyst protonates the carbonyl group of benzoyl chloride. Then, the nucleophilic attack of amino group of 2-aminobenzamide to carbonyl group of benzoyl chloride resulted in the formation of benzamides 3. The following nucleophilic attack of NH2 to carbonyl group of compound 3 and subsequent dehydration afforded quinazolinone derivatives 4 (Scheme 2)24.
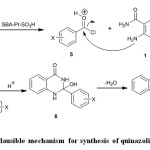 |
Scheme 2: Plausible mechanism for synthesis of quinazolinones 4a-e Click here to View figure |
SBA-Pr-SO3H with its distinct features such as the pore size of 6 nm, acts as an efficient nano-reactor in this reaction (Fig. 2). Fig. 2 also illustrates the SEM and TEM images of SBA-Pr-SO3H. SEM image (Fig. 2, a) shows uniform particles about 1m. The same morphology was observed for SBA-15. It can be concluded that morphology of solid was saved without change during the surface modifications. On the other hand, the TEM image (Fig. 2, b) reveals the parallel channels, which resemble the pores configuration of SBA-15. This indicates that the pore of SBA-Pr-SO3H was not collapsed during two-steps reactions.
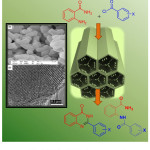 |
Fig. 2: SBA-Pr-SO3H acts as a nano-reactor Click here to View figure |
The sulfonic acid functionalized SBA-15 was usually synthesized through direct synthesis or post-grafting25-26. A schematic illustration for the preparation of SBA-Pr-SO3H was shown in Fig. 3. First, the calcined SBA-15 silica was functionalized with (3-mercaptopropyl)trimethoxysilane (MPTS) and then, the thiol groups were oxidized to sulfonic acid by hydrogen peroxide (Fig. 3).
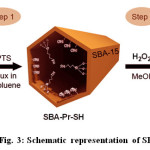 |
Fig. 3: Schematic representation of SBA-Pr-SO3H |
CONCLUSION
In conclusion, we have developed an efficient and mild procedure for the synthesis of a series of quinazolinone derivatives via the reaction of corresponding benzoyl chlorides and 2-aminobenzamide. The use of SBA-Pr-SO3H in this reaction has the advantages of being reusable and environmentally benign nano-reactor that the reaction proceeds easily in its nano-pores.
ACKNOWLEDGEMENTS
We gratefully acknowledge for financial support from the Research Council of Alzahra University and University of Tehran.
REFERENCES
- Mulakayala N., Kandagatla B., Ismail Rapolu R. K., Rao P., Mulakayala C., Kumar C. S., Iqbal J. and Oruganti S. Bioorg. Med. Chem. Lett., 22: 5063 (2012).
- Adib M., Ansari S., Mohammadi A. and Bijanzadeh H. R. Tetrahedron Lett., 51: 30 (2010).
- Gellibert F., Fouchet M. H., Nguyen V. L., Wang R., Krysa G., de Gouville A. C., Huet S. and Dodic N. Bioorg. Med. Chem. Lett., 19: 2277 (2009).
- Khan M. T. H., Khan R., Wuxiuer Y., Arfan M., Ahmed M. and Sylte I. Bioorg. Med. Chem., 18: 4317 (2010).
- J. Azizian, A. Shameli, S. Balalaie, M. M. Ghanbari, S. Zomorodbakhsh, M. Entezari, S. Bagheri and G. Fakhrpour., Orient. J. Chem., 28(1), 327-332 (2012)
- Tavakoli-Hoseini N. and Davoodnia A. Chin. J. Chem., 29: 1685 (2011).
- Mhaske S. B. and Argade N. P. Tetrahedron, 62: 9787 (2006).
- Davoodnia A., Allameh S., Fakhari A. R. and Tavakoli-Hoseini N. Chin. Chem. Lett., 21: 550 (2010).
- Zhou J. and Fang J. J. Org. Chem., 76: 7730 (2011).
- Roopan S. M., Maiyalagan T. and Khan F. N. Can. J. Chem., 86: 1019 (2008).
- Wang X-S., Yang K., Zhang M-M. and Yao C-S. Synth. Commun., 40: 2633 (2010).
- Chen J., Wu D., He F., Liu M., Wu H., Ding J. and Su W. Tetrahedron Lett., 49: 3814 (2008).
- Tavakoli-Hoseini N. and Davoodnia A. Synth. React. Inorg. Met. Org. Chem., 42: 76 (2012).
- Mohammadi Ziarani G., Lashgari N. and Badiei A. Sci. Iran., 20: 580 (2013).
- Mohammadi Ziarani G., Badiei A., Mousavi M., Lashgari N. and Shahbazi A. Chin. J. Catal., 33:1832 (2012).
- Mohammadi Ziarani G., Badiei A., Dashtianeh Z., Gholamzadeh P. and Hosseini Mohtasham N. Res. Chem. Intermed., 39: 3157 (2013).
- Bahrami K., Khodaei M. M. and Fattahpour P. Catal. Sci. Technol., 1: 389 (2011).
- Mohammadi Ziarani G., Badiei A., Khaniania Y. and Haddadpour M. Iran. J. Chem. Chem. Eng., 29: 1 (2010).
- D. S. Ande., Orient. J. Chem., 28(2), 975-978 (2012)
- Wang G. W., Miao C. B. and Kang H. Bull. Chem. Soc. Jpn., 79: 1426 (2006).
- Paterson T. M., Smalley R. K. and Suschitzky H. Synthesis, 187 (1975).
- Mehta P. Indian. J. Chem., 9: 109 (1971).
- Zentmyer D. T. and Wagner E. C. J. Org. Chem., 14: 967 (1949).
- Potewar T. M., Nadaf R. N., Daniel T., Lahoti R. J. and Srinivasan K. V. Synth. Commun., 35: 231 (2005).
- Lim M. H., Blanford C. F. and Stein A. Chem. Mater., 10: 467 (1998).
- Wight A. P. and Davis M. E. Chem. Rev., 102: 3589 (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.









