Macrobenthic diversity in Horseshoe Crab nesting ground - Balok station, Pahang, Malaysia
Akbar John, B., Jalal, K.C.A., Kamaruzzaman, B.Y.
Institute of Oceanography and Maritime studies (INOCEM), Kulliyyah of Science, International Islamic University Malaysia, Jalan Sultan Ahmad Shah, Bandar InderaMahkota, 25200, Kuantan Pahang, Malaysia
DOI : http://dx.doi.org/10.13005/ojc/290406
Article Received on :
Article Accepted on :
Article Published : 09 Jan 2014
A complete year data on the diversity and distribution of major macrobenthic community along the horseshoe crab nesting ground in Balok station was studied. Here we present the monthly and seasonal variation in the macrobenthic community along the balok station during full moon days. Monthly variation in the diversity of macrobenthos at Balok station during full moon days showed that Highest diversity during June 2010 (Shannon H’ = 0.673; Simpson 1/D = 4.554) followed by Sep-10 (H’ = 0.663; 1/D = 4.431) while the lowest diversity was recorded during Mar-10 (H’ = 0.545; 1/D = 2.916). In general, there was a significant variation in the macrobenthic diversity was observed between monsoon and non-monsoon period (p < 0.05). Margaleff and Mackintosh richness indexes showed that the species richness was higher during Feb-2011 (Marfalf d = 1.748; McIntosh D = 1.067) followed by Dec-10 (d = 1.691; D = 1.063) and Nov-10 (d = 1.655; D = 1.06). The macrobenthic richness was lower during May-10 (d = 1.28; D = 1.027). As observed in diversity of Macrobenthos, richness was also higher during monsoon period compared to non monsoon time. Berger-Parker Dominance (1/d) index showed that species dominance was higher during June-10 (1/d = 3.795) followed by Sep-10 (1/d = 3.757) and it was lower during Mar-10 (1/d = 2.109). There was no significant variation in the evenness was observed during sampling period which showed the homogeneous distribution of species round the year. Shannon diversity index value (H’ = < 1.1) clearly showed that the macrobenthic diversity along the nesting grounds of horseshoe crabs are under greater pressure.
KEYWORDS:Horseshoe crab;Macrobenthos;Nesting ground;Balok station;Diversity Indices
Download this article as:| Copy the following to cite this article: Akbar J. B, Jalal K.C.A, Kamaruzzaman B.Y. Macrobenthic diversity in Horseshoe Crab nesting ground - Balok station, Pahang, Malaysia. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Akbar J. B, Jalal K.C.A, Kamaruzzaman B.Y. Macrobenthic diversity in Horseshoe Crab nesting ground - Balok station, Pahang, Malaysia. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1535 |
Introduction
Macrobenthos in marine sediment play an important role in ecosystem processes such as nutrient cycling, pollutant metabolism, dispersion and burial, and in secondary production (Snelgrove, 1998). Structure of benthic community is now frequently used in pollution effect monitoring programmes. Limited benthic studies have been conducted in the tropics compared to higher latitudes and the theory relating to the community structure is based largely on the studies from temperate regions (Alongi, 1990). It is important to establish baseline for tropical regions and improve our understanding of biodiversity in marine environment. Benthic animals are found at all depths and are associated with all substrates. About 80% of the benthic organisms are Epifauna that are living on or are attached to the surface of rocky areas or firm sediments. Animals that live buried in the substrate belong to the Endofauna (infauna) and are commonly associated with soft sediments such as sand or mud. Some animals of the sea floor are sessile as adults (Cirripedia, Anthozoa, Bivalvia), while others are permanently motile (Crustacea, Echinodermata, and other Mollusks). Most benthic forms produce motile larvae that spend a few weeks of their live cycles as meroplankton (biphasic life-cycle), allowing the species to avoid overcrowding and to colonize new areas. Benthos can also be sorted according to their sizes. Basically, benthos with size between 500-1000 µm is called Macrobenthos (Tagliapietraet al., 2009). Assemblages of benthic macro-organisms including mollusks, echinoderms, and polychaetes, were associated with each characteristic type of sediment on continental shelves around the world at all latitudes. Despite being benthic feeders, they also possess numerous characteristics that enable them to serve as water quality indicators. These organisms are diverse, abundant, broadly distributed, and have successfully exploited most aquatic habitats (Merritt et al., 2008).
Virtually, no previous studies were conducted to determine the macrobenthic assemblage in horseshoe crab nesting grounds along both Malaysian coast. Hence a comprehensive study covering both monsoon and nonmonsoon seasonal major macrobenthic community assemblage along the observed nesting ground of horseshoe crabs was conducted. Here we present the diversity and distribution of macrobenthic community in the Balok horseshoe crab nesting ground observed during full moon days of both monsoon and non monsoonal period.
Materials and Methods
Study Area Description
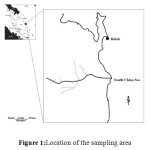 |
Figure 1:Location of the sampling area |
Balok (Lat3°56.194’ N, Long103°22.608’ E) in the Pahang state of East coast of Malaysia were observed to be the nesting ground of Horseshoe crabs (Zalehaet al., 2010; Akbar John et al., 2011 & 2012; Zaleha et al., 2012). Adult horseshoe crabs migrate from the off shore continental shelf to spawn on intertidal sandy (T.gigas) and mud sandy beaches and mangrove area (C. rotundicauda) at every full and new moon at these sampling locations (Figure 1). The sediments of the sampling location were observed to be soft which may contribute to the ease of laying eggs and egg burial by the female horseshoe crab. Meteorological data showed that high rainfall during monsoon season (November to January). Twenty years (1968 to 1987) of accumulated data obtained from the Malaysian Meteorological Department (MMD) showed that the monsoon seasons with strong winds and long frequency periods with mean annual rainfall of 3064mm occurred from November to January. Meanwhile the non-monsoon seasons with low rainfall occurred during April, May and June. Field observation unveiled the intensive fishery activities along the sampling locations besides industrial activities and human inhabitation processes which could possibly introduce considerable level of contaminants into the water body.
Sample Collection and Preparation
Macrobenthic samples were collected from the nesting grounds of horseshoe crabs at Balok station in every full moon days between March 2010 and February 2011. A total of 10 sites were sampled from Balok beach (Sites were selected depends on the total length of nesting grounds in each sampling locations). Macrobenthic samples were collected in nesting grounds using hand scoop from both the sampling locations (Balok = 10 sampling sites) during low tide time.
Macrobenthos Identification
The sediment samples were sieved through electrical shaker using 0.5mm mesh size sieve as a final layer to collect the macrobenthic organisms. The retained macrobenthos were collected using smooth forceps and placed in appropriate vials containing 70% ethanol (Gurr, 1965) in order to preserve the samples. Samples were identified using standard references (Arnold and Britles, 1989).
Diversity Indices
Shannon Diversity Index
This index is an index applied to biological systems by derived from a mathematical formula used in determining the heterogeneity of the samples (Mandaville, 2002). It’s the most preferred index among the other diversity indices. The index values are between 0.0 – 5.0. Results are generally within the range of 1.5 and 3.5, and it rarely exceeds 4.5. The values above 3.0 indicate that the structure of habitat is stable and balanced; the values under 1.0 indicate that there are pollution and degradation of habitat structure.
Formula
H’ = -Σ [(ni / N) x (lnni / N)]
H’: Shannon Diversity Index
ni: Number of individuals belonging to i species
N: Total number of individuals
Simpson Diversity Index
It’s a diversity indices derived by Simpson in 1949 (Mandaville 2002). Simpson index values (∆) are between 0 – 1. But while calculating, final result is subtracted from 1 to correct the inverse proportion.
Formula
1 – D = [Σ ni (ni -1)] / N (N-1)
D: Simpson Diversity Index
ni: Number of individuals belonging to i species
N: Total number of individuals
Here I have adopted the reciprocal form (1/D) of Simpson index for data interpretation.
Margalef Diversity Index
It has no limit value and it shows a variation depending upon the number of species. Thus, it’s used for comparison the sites (Boyle et al., 1995).
Formula
d = (S-1) / ln N
d: Margalef Diversity Index
S: Total number of species
N: Total number of individuals
Mcintosh Diversity Index
McIntosh (1967) proposed that the community can be envisaged as a point in an S-dimensional hyper volume and that the Euclidean distance of the assemblage from its origin could be used as a measure of diversity. The distance is known as U and it is calculated as:
![]()
The McIntosh U index is not formally a dominance index. However, measures of diversity (D) or dominance that is independent of N can also be calculated;
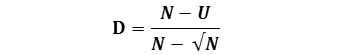
And a further evenness measures can be obtained from the formula as given below
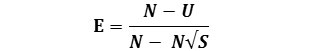
Berger‐Parker Index
This simple intrinsic index expresses the proportional importance of the most abundant species. As with the Simpson index, the reciprocal form of the Berger-Parker index is usually adopted so that an increase in the value of the index accompanies an increase in diversity and a reduction in dominance (Berger and Parker, 1970). The formula used to calculate Berger‐Parker dominance index was
d=Nmax/N
Results
The most dominant macrobenthic invertebrates observed at the sampling stations are bivalves (Anadaragranosa, A.antiquata, Circe sp., Tellina virgate, Tellina sp., Donaxcuniatus, Donaxvariabilis, Mycdorastriata and Codakia orbicularis), gastropods (Cerithidea cingulate, C. obtuse, Cerithiumlitteratum, Neritina sp., Littorinascabra, L.fasiata, L.undulata, Clitonoualaniensis and Umboniumvestiarum), polychaetes (Ganoderma austral, Lentinussquarrosulus, Microporellus sp., Pycnoporussanguineus, Rigidoporus sp., Lenziteselegans, Microporus sp. and Trametes sp.) and crustaceans (Juvenile Penaeidshirimp, Juvenile forms of common crabs and hermit crabs).
Monthly variation in the diversity of macrobenthos at Balok station during full moon days is shown in Table 1. Highest diversity of macrobenthic community was observed during June 2010 (Shannon H’ = 0.673; Simpson 1/D = 4.554) followed by Sep-10 (H’ = 0.663; 1/D = 4.431) while the lowest diversity was recorded during Mar-10 (H’ = 0.545; 1/D = 2.916). In general, there was a significant variation in the macrobenthic diversity was observed between monsoon and non-monsoon period (p > 0.05). Margaleff and Mackintosh richness indexes showed that the species richness was higher during Feb-2011 (Marfalf d = 1.748; McIntosh D = 1.067) followed by Dec-10 (d = 1.691; D = 1.063) and Nov-10 (d = 1.655; D = 1.06). The macrobenthic richness was lower during May-10 (d = 1.28; D = 1.027). As observed in diversity of Macrobenthos, richness was also higher during monsoon period compared to non monsoon time. Berger-Parker Dominance (1/d) index showed that species dominance was higher during June-10 (1/d = 3.795) followed by Sep-10 (1/d = 3.757) and it was lower during Mar-10 (1/d = 2.109). There was no significant variation in the evenness was observed during sampling period which showed the homogeneous distribution of species round the year. But slight variation in species evenness was noted between monsoon and non monsoon period.
The four main groups of macrobenthic fauna found in the horseshoe crab nesting grounds at Pahang coast are Bivalves, Gatropoda, Crustacea and Polychaeta as mentioned earlier. Besides these major Macrobenthos, there were other miscellaneous groups were also found covering including insects, amphipods, Isopods, larval and juvenile stages of fishes, foraminifera and Annelidan worms etc. Among the macrobenthos, Bivalves were the most community encountered throughout the sampling periods in both the stations. They accounted for 37.2% and 31.5% at Non monsoon and monsoon season during new moon days and 28% and 21.5% at full moon days in Balok station respectively (Table 2). Gastropods were the second dominant macrobenthic community followed by other organisms (including insects, amphipods, Isopods, larval and juvenile stages of fishes, foraminifera and Annelidan worms) and polychaetes. Crustaceans were of least dominant in both the sampling locations. In general, the macrobenthic community composition was higher during full moon days compared to new moon period.
Species Similarity Index
Bray-Curtis single linked cluster analysis was performed to determine the percentage of species similarity within the sampling station during sapling period (Figure 2). The constructed dendogram showed apparent 2 clusters segregating monsoon and non monsoon seasons. Highest percentage species similarity was observed between March and April-2010 (93.8486%) followed by July and May-2010 (90.826%) during non monsoon period. On the other hand, highest percentage species similarity was observed between Nov-10 and Dec-10 (90.0609%) followed by Nov-10 and Jan-2011 (88.3959%) during monsoon period. The lowest percentage species similarity was recorded between March-10 and October-10 (45.1276%) during non monsoon and between January-11 and February-11 (74.7592%) during monsoon time. There was a significant variation in the species similarity was observed between monsoon and non monsoon periods (p < 0.05).
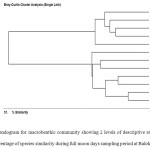 |
Figure 2: Dendogram for macrobenthic community showing 2 levels of descriptive resolution showing percentage of species similarity during full moon days sampling period at Balok station. Click here to View figure |
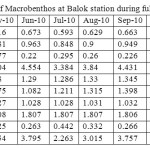 |
Table 1:Monthly variation in the diversity of Macrobenthos at Balok station during full moon period. Click here to View table |
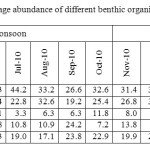 |
Table 2: Monthly variation in the percentage abundance of different benthic organisms at Balok during full moon days. Click here to View table |
Discussion
The role and importance of macrobenthos in the marine ecosystem has long been known and discussed. They form a major food item for the bottom feeders like demersal fishes. Moreover, certain macrobenthic species are themselves of commercial importance as, for example, the prawns, crabs and cockles. It was also evident that human disturbance in marine ecosystems could possibly be accessed directly from physical and chemical parameters (Daskalakis and O’Connor, 1995), or indirectly using communities of macrobenthic organisms that characterize the ecological quality of their habitats (Currie and Isaacs, 2005). Macrobenthic species are of special interest in this context because most of them are sessile or have a limited mobility (Olsgard and Gray, 1995). In general, Macrobenthic diversity was comparatively lower during full moon (FM) days. Overall, macrobenthic diversity was higher during June, 2010
This observation is well coincided with the mating season of horseshoe crab at both these station). Kassimet al. (2008) and John et al. (2010) had found that the peak mating season of horseshoe crabs along the Pahang coast was during June-August. There was a significant variation in the macrobenthic diversity was observed between monsoon and non monsoon period (p < 0.05) in Balok station. This might probably due to the 1. Differences in the relative contributions of individual discriminating species along the Balok coast. Positive relationship between the abundance of benthic fauna and concentration of organic carbon in sediments had been documented by many workers (Hyland et al., 1991; Diaz and Rosenberg, 1995). Middelburg and Herman, (2007) has found that estuaries receive higher amount of organic load compared to other coastal area. Macrobenthos, richness was also higher during monsoon period compared to non monsoon time. This might be due to the high stress tolerance of representative species of the major macrobenthic groups during monsoonal cycle. However, the density of theserepresentative species is lower compared to non monsoonal period. Bakus (1990) also stated that the effect of various factors such as depth, sediment grain size, salinity and predation density are factors in controlling the population density of macrobenthos and the most important factors are sediment size and predator density. Another indirect factor for faunal distribution and abundance is the amplitude and the direction of monsoonal wind and the shifting of the monsoon have a considerable impact in terms of sediment disturbance, this would have an effect directly on the density and diversity of macrobenthic fauna (Hylleberget al., 1985). There was no significant variation in the evenness was observed during sampling period in both the sampling stations which showed the homogeneous distribution of species round the year.
Detailed analysis of species similarity matrix within the sampling area indicates that adjacent sites had higher similarity on average than site pairs further apart. Similar observation was noted in macrobenthic community observed in soft sediments (Ellingsen, 2001; Van Hoeyet al., 2005). Overall, Shannon diversity index value (H’ = < 1.1) clearly showed that the macrobenthic diversity along the nesting grounds of horseshoe crabs are under greater pressure. Various anthropogenic and coastal developmental activities including construction of resorts, fishing, and pollution input might have played an important role in the reduction of macrobenthic diversity along the Pahang cost. Field observation showed that the shore reaching horseshoe crab population had drastically reduced in recent years due to poor diversity of major prey item besides notable degradation of habitat structure. Similar observation was noted along the Japan coast where habitat degradation and destruction are primarily responsible for the shrinking of breeding zones of horseshoe crab population (Iwaoka and Okayama, 2009; Tanacrediet al., 2009).
References
- Akbar John, B., Kamaruzzaman, B.Y., Jalal, K.C.A., Zaleha, K. (2012). Sediment Profiling of the Nesting Grounds of Horseshoe Crabs at East Peninsular Malaysia.International Journal of Biology. 4 (2): 159-165.
- Akbar John, B., Kamaruzzaman, b.y., Jalal, K.C.A., Zaleha.K. (2011). Hydrology of the horseshoe crab nesting grounds at Pahang coast, Malaysia. Oriental Journal of Chemistry. 27 (4): 1475-1483.
- Alongi, D. M. (1990). The ecology of tropical soft-bottom benthic ecosystems. Oceanogrphy and Marine Biology Annual Review 28, 381-496.
- Arnold, P. W., & Britles, R. A. (1989). Soft-sediment Marine Invertebrates of Southeast Asia and Australia: A Guide to Identification. (pp. 227). Townsville: Australian Institute of Marine Science.
- Bakus, G. J. (1990). Quantitative ecology and marine biology: Oxford & IBH Pub. Co
- Berger, W. H., & Parker, F. L. (1970 ). Diversity of planktonic foraminifera in deep-sea sediments. Science, 168(3937), 1345-1347.
- Boyle, T. J. B., & Boontawee, B. (1995). Measuring and monitoring biodiversity in tropical and temperate forests: proceedings of a IUFRO symposium held at Chiang Mai, Thailand, August 27th – September 2nd, 1994: Center for International Forestry Research.
- Currie, D. R., & Isaacs, L. R. (2005). Impact of exploratory offshore drilling on benthic communities in the Minerva gas field, Port Campbell, Australia. Marine Environmental Research, 59(3), 217-233.
- Daskalakis, K. D., & O’Connor, T. P. (1995). Normalization and Elemental Sediment Contamination in the Coastal United States. Environmental Science & Technology, 29(2), 470-477.
- Diaz, R. J., & Rosenberg, R. (1995). Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanography and Marine Biology: an Annual Review, 33, 245-303.
- Ellingsen, K. E. (2001). Biodiversity of a continental shelf soft-sediment macrobenthos community. Marine Ecology Progress Series, 218, 1-15.
- Gurr, E. (1965). The rational use of dyes in biology and general staining methods: Williams and Wilkins.
- Hyland, J., Baptiste, E., Campbell, J., kennedy, J., Kropp, R., & Williams, S. (1991). Macroinfaunal communities of the Santa Maria basin on the california outer continental shelf and slope. Marine ecology progress series, 78, 147-161.
- Hylleberg, J., Nateewathana, A., & Chatananthawei, B. (1985). Temporal changes in sediment characteristics on the west coast of Phuket Island. Phuket Marine Biological Center Research Bulletin., 37, 16
- Iwaoka, C., & Okayama, T. (2009). Public Awareness and Community-Based Conservation for the Horseshoe Crab at Saikai National Park in Nagasaki Prefecture, Japan. In J. T. Tanacredi, Botton, M.L., Smith, D.R., Earle, S.A. (Ed.), Biology and Conservation of Horseshoe Crabs (pp. 571-583). US: Springer.
- John, B. A., Jalal, K. C. A., Kamaruzzaman, B. Y., & Zaleha, K. (2010). Mechanism in the clot formation of horseshoe crab blood during bacterial endotoxin invasion. Journal of Applied Sciences, 10(17), 1930-1936.
- Kassim, Z., Shahuddin, H., Shaharom, F., & Chatterji, A. (2008). Abundance of three species of the horseshoe crab along the coast of Malaysia. Journal of the Bombay Natural History Society, 105, 209-211.
- Mandaville, S. M. (2002). Benthic Macroinvertebrates in Freshwater – Taxa Tolerance Values, Metrics, and Protocols, . Project H -1. (Nova Scotia: Soil & Water Conservation Society of Metro Halifax)
- McIntosh, R. P. (1967). An Index of Diversity and the Relation of Certain Concepts to Diversity. Ecology, 48(3), 392-404.
- Merritt, R. W., Holzenthal, R. W., Cummins, K. W., & Berg, M. B. (2008). An introduction to the Aquatic insects of North America: Kendall/Hunt Pub. Co.
- Middelburg, J. J., & Herman, P. M. J. (2007). Organic matter processing in tidal estuaries. Mar. Chem., 106(1-2), 127-147.
- Olsgard, F., & Gray, J. S. (1995). A comprehensive analysis of the effects of off shore oil and gas exploration and production on the benthic communities of the Norwegian continental shelf. Marine Ecology Progress Series, 122, 277-306.
- Snelgrove, P. V. R. (1998). The biodiversity of macrofaunal organisms in marine sediments. Biodiversity and Conservation, 7(9), 1123-1132.
- Tagliapietra, D., Sigovini, M., & Ghirardini, A. V. (2009). A review of terms and definitions to categorise estuaries, lagoons and associated environments. Marine and Freshwater Research, 60, 497–509.
- Tanacredi, J. T., Botton, M. L., & Smith, D. (2009). Biology and Conservation of Horseshoe Crabs: Springer.
- Van Hoey, G., Vincx, M., & Degraer, S. (2005). Small- to large-scale geographical patterns within the macrobenthic Abra alba community. Estuarine, Coastal and Shelf Science, 64(4), 751-763.
- Zaleha, K., Akbar John, B., ErniAtika, H., Kamaruzzaman, B.Y. Jalal, K.C.A. (2012). Spawning and nesting behaviour of Tachypleusgigas along the east coast of Peninsular Malaysia. International Journal of Biology. 4 (2): 102-111.
- Zaleha, K., Kamaruzzaman, B. Y., John, B. A., & Ong, M. C. (2010). Cd, Cu and Pb Concentration Levels in Horseshoe Crab Nesting Grounds of Pahang Coast, Malaysia. Journal of Biological Sciences, 10(8), 790-794.

This work is licensed under a Creative Commons Attribution 4.0 International License.









