Diazo-Coupling: A Facile Mean for the Spectrophotometric Determination of Rasagiline hemitartrate
Muvvala Subrahmanya Sudhir1 and Ratnakaram Venkata Nadh 2*
1Department of Chemistry, S. S. & N. College, Narasaraopet–522 601, India. 2* School of Biotechnology, Vignan University, Vadlamudi-522213, India.
DOI : http://dx.doi.org/10.13005/ojc/290429
Article Received on :
Article Accepted on :
Article Published : 16 Jan 2014
A simple, precise, accurate, high reproducible and economical visible spectrophotometric method of analysis for the synthesized rasagiline hemitartrate was developed and validated. The proposed method involves diazotization of sulphanilic acid under acidic conditions in presence of sodium nitrite, followed by its coupling with rasagiline in alkaline medium. The absorption spectra of the yellow colored chromogen formed between rasagiline and positive diazonium ion has absorption maximum at 440 nm. The linear regression analysis data for the calibration plot showed good linear relationship (r = 0.99937) with in the concentration range of 0 – 10 μg mL-1. The limit of detection and limit of quantitation were found to be 0.033 μg mL-1and 0.1 μg mL-1 respectively. This method was tested and validated for various parameters according to ICH guidelines. The results demonstrated that the procedure is accurate, precise and reproducible (R.S.D. < 2 %).
KEYWORDS:Rasagiline Hemitartrate; Sulphanilic Acid; Diazocoupling; Validation; Estimation; Visible Spectrophotometry
Download this article as:| Copy the following to cite this article: Sudhir M. S, Nadh R. V. Diazo-Coupling: A Facile Mean for the Spectrophotometric Determination of Rasagiline hemitartrate. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Sudhir M. S, Nadh R. V. Diazo-Coupling: A Facile Mean for the Spectrophotometric Determination of Rasagiline hemitartrate. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1735 |
INTRODUCTION
R(+)-N-propargyl-1-aminoindan (“R-PAI”) is also known as rasagiline and is a chiral compound with one asymmetric carbon atom in a five membered ring with an absolute (R) configuration which is produced as single enantiomer1. Rasagiline is a propargylamine-based drug indicated for the treatment of idiopathic Parkinson’s disease2. In addition to rasagiline base, its acid addition salts (viz., mesylate, maleate, fumarate, tartrate, hydrobromide, p-acetate, benzoate, phosphate, tolunesulfonate and sulfate) are pharmaceutically acceptable3. Though different methods were reported for the determination of rasagiline mesylate in bulk and in pharmaceutical dosage forms4-7, only one each of RP-HPLC8 and UV spectrophotometric method9 were reported for the determination of rasagiline hemitartrate.
In view of a lack of a commercial supplier and non development of visible spectrophotometric assay method till the date for rasagiline hemitartrate, the present study is aimed at its synthesis and development of a visible spectrophotometric analytical method for its bulk form by conducting systematic trials.
MATERIALS AND METHODS
Systronics digital spectrophotometer (model-106), Shimadzu AUX-220 and an Elico LI-120 digital pH meter were used. Chemicals used were of analytical reagent grade and Milli–Q water was used throughout the investigation.
0.6% (w/v) Sulphanilic acid (4-aminobenzenesulfonic acid) solution: This solution was prepared by dissolving 0.6 g of sulphanilic acid in 75 mL of hot distilled water and diluted with 25 mL of acetic acid in a 100 mL volumetric flask. The solution was stored in a brown-coloured bottle.
Synthesis and characterization of Rasagiline hemitartrate: The procedure adopted was in accordance our earlier report8.
RESULTS AND DISCUSSION
Absorption spectrum of coloured complex
Into a 10 mL volumetric flasks, 1 mL of 0.6% sulphanilic acid solution and 1 mL of 3 N hydrochloric acid solution were added. After cooling the contents to 40C in an ice bath, 1 mL of 3% sodium nitrite was added and shaken for three minutes. To remove the added excess sodium nitrite, 1 mL of 4% sulphamic acid solution was added and shaken for two minutes. Then an aliquot of working standard drug solution (in the range 2 – 10 mg) was added and the medium was made alkaline by the addition of 2 mL of 10% sodium hydroxide solution. The contents were made upto mark with distilled water to obtain yellow colored solution. The developed chromophore was scanned within the wavelength region of 400 – 800 nm against a blank solution. The resulting spectrum was shown in Figure 1, and the absorption curve showed characteristic absorption maxima at 440 nm.
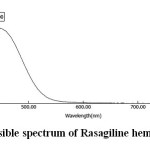 |
Fig. 1: Visible spectrum of Rasagiline hemitartrate Click here to View figure |
Theory of absorption spectrum of coloured complex
Coupling of diazonium ion with drug molecules in basic medium to form azodyes has been used for the estimation of drugs by researchers10,11. In the proposed method, chromogen (diazonium salt) was prepared by diazotization of sulphanilic acid (having amino group) with sodium nitrite in presence of hydrochloric acid. Then this coloring agent coupled to rasagiline to form an yellow colored complex in alkaline medium.
The absorption maximum for the coloured complex was found to be 440 nm. The absorption maxima of aqueous solutions of pure rasagiline and sulphanilic acid were 264 and 249 nm respectively. The lmax of diazonium salt of sulphanilic acid was 270 nm. The blank solution (prepared on addition of sodium hydroxide to this diazonium salt of sulphanilic acid) didn’t shift in the absorption maximum. However, a remarkable shift in the absorption maximum was observed when the blank solution was added to the drug solution. This was due to the formation of coupling product, which exhibits an absorption band peaking at 440 nm. This reaction was exploited to develop a spectrophotometric method for the determination of rasagiline hemitartrate in bulk drug form.
Mechanism of the color reaction
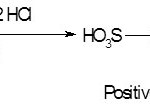
The primary aromatic amines (p – nitro aniline / sulphanilic acid) react with NaNO2 in acid medium in the temperature range of 0-30C to give diazonium salt12. Colored azo compounds are formed by the coupling of the diazonium salts with strong nucleophiles (like electron rich aromatic / heteroaromatic compounds)13. The diazocoupling reaction can be considered as a proton eliminating condensation of a positive diazonium ion with another compound possessing an active hydrogen atom.
The proposed method is based on the reaction of diazotization of amino group bearing sulphanilic acid (4-amino benzene sulphonic acid) with sodium nitrite in presence of hydrochloric acid at a temperature of 3°C to form an electrophile, i.e., positive diazonium ion. The reaction is usually carried out in an ice bath and the excess sodium nitrite was removed by treatment with sulphamic acid. Since most of the diazonium salts are unstable11, the diazonium salt was used immediately.
 |
Click here to View figure |
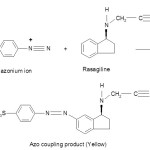
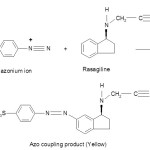
In alkaline medium, the positive diazonium ion couples at third position in rasagiline to form an yellow coloured azo product.
 |
Click here to View figure |
Optimization of reactions conditions
Factors affecting the reaction conditions (concentrations of sulphanilic acid, hydrochloric acid, sodium nitrite, sulphamic acid and temperature) were studied by altering each variable in turn while keeping the others constant and the optimum conditions were established. The optimum conditions were selected based on their ability to give maximum absorbance and were maintained throughout the studies.
In 1858, Peter Griess discovered the diazotization reaction14 which requires three key components viz., an arylamine (Sulphanilic acid), a mineral acid (hydrochloric acid) and a source of nitrous acid (sodium nitrite)15.
–O3SC6H4NH2 + 2 HCl + NaNO2 → –O3SC6H4N2Cl + NaCl + 2H2O
As per the above equation, two equivalents of hydrochloric acid are required for sulphanilic acid. However, addition of excess acid is suggested to avoid the triazen formation. Triazen forms by the reaction of diazotized sulphanilic acid with the free sulphanilic acid15.
 |
Click here to View figure |
Above 0.05N, with an increase in hydrochloric acid concentration, the rate of diazotization of sulphanilic acid increases16. Hence, the hydrochloric acid concentration was varied in between 0.05 to 1.5 N and the diazotization was found to be completed within three minutes for concentrations of 0.5 N and above up to 1.5 N. Hence, 1.0 N hydrochloric acid concentration was maintained in diazotization mixture by the addition of 1mL of 3 N HCl. Mixing of diazotization contents was done for six minutes though, the reaction completes within three minutes. The extended mixing is required because the reaction with nitrous acid is very slow towards the end of the diazotization17. To determine the optimum concentration of sulphanilic acid, the absorbance was studied by the addition of fixed volume (1 mL) of sulphanilic acid solution with variable concentrations (0.05–0.8%). Satisfactory results were obtained with 1 mL of a 0.6% sulphanilic acid solution.
Carrying out the diazotization at low temperature is advantageous18 due to (a) enhanced solubility of free nitrous acid which prevents the escape of nitrous gases from the acid medium and (b) improved stability of the diazotized sulphanilic acid (as the diazotized sulphanilic acid degrades to p–hydroxybenzene sulphonic acid at higher temperatures). Therefore, diazotization was carried out below 30C. The effect of sodium nitrite concentration on diazotization was studied by the addition of 1 mL of NaNO2 solutions with variation in the concentration range of 0.3 – 5%. An increase in absorbance was observed with an increase in sodium nitrite concentration and became constant at 1%, above which, absorbance remained constant up to 5%. Therefore 1 mL of 3% sodium nitrite was chosen as an optimum value for the determination studies.
Unlike hydrochloric acid, addition of excess sodium nitrite should be avoided due to destabilization of the diazotized salt by the surplus amount of nitrous acid produced in the medium18. Development of immediate blue colouration by moist potassium iodide starch paper helps the detection of surplus nitrous acid17. After completion of diazotization, the left over sodium nitrite can be destroyed by the addition of either urea or sulphamic acid19. These reagents convert the excess nitrite into nitrogen gas in acidic medium20. Sulphamic acid was preferred to remove the excess nitrite as it reacts faster than urea21.
NH2CONH2 + 2HNO2 → CO2 + 2N2 + 3H2O
HOSO2NH2 + HNO2 → H2SO4 + N2 + H2O
By knowing the leftover nitrite after diazotization, the amount of sulphamic acid to be added for its removal can be calculated from the stoichiometry of the respective destruction reaction. It was found to be 1 mL of 4% sulphamic acid.
The effect of the addition of sodium hydroxide to the diazocoupling mixture was studied by following absorbance. The volumes of 10% sodium hydroxide varied from 1.0 to 3 mL. It was observed that maximum absorbance was observed by the addition of 2.0 ± 0.5 mL. A decrease in absorbance beyond 2.5 mL can be attributed to the partial decolorization of the dye at higher concentrations of alkali22. A decrease in colour intensity of the mixture at higher alkali conditions can be explained by consideration of acid–base equilibriums of diazonium compounds23. In general, aryldiazonium cations (e.g., phenyldiazonium) can lose their positive charge (i.e., high electrophilicity) and form diazohydroxides due to the attachment of anion to the terminal nitrogen atom. Further increase in alkalinity leads to subsequent deprotonation to form diazotates.
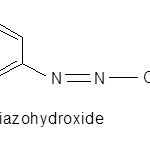 |
Click here to View figure |
Hence, in the present case, a maximum absorbance was observed when reaction mixture maintained at 0.5 M sodium hydroxide. In spite of rapid development of the yellow colored azo-dye, a maximum absorbance was attained after about 2 min at room temperature and the colour intensity was quite stable for at least one hour.
Validation of Method
For analytical determination of drugs the most important step is validation. Linearity and range, accuracy and precision, recovery, ruggedness, limit of detection (LOD) and limit of quantitation (LOQ) are the main validation parameters24.
Linearity and range: The calibration graph was found to be linear in the range of 0 – 10 μg mL-1 for the proposed method (Figure 2). Each point of the calibration graph was the mean value acquired from three independent measurements (Table 1). The linear regression equation was y = 0.01195 x + 0.09944. The various optical and regression parameters were given in Table 2.
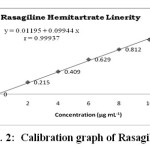 |
Fig. 2: Calibration graph of Rasagiline Click here to View figure |
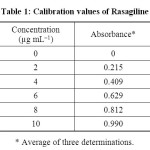 |
Table 1: Calibration values of Rasagiline Click here to View table |
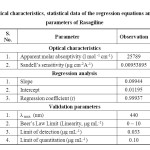 |
Table 2: Optical characteristics, statistical data of the regression equations and validation parameters of Rasagiline Click here to View table |
Accuracy: The mean of percentage recovery values were given in Table 3. Low values of standard deviation and relative standard deviation confirm a high level of accuracy for the proposed method.
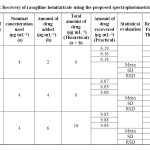 |
Table 3: Recovery of rasagiline hemitartrate using the proposed spectrophotometric method Click here to View table |
Precision: The precision of the method was satisfactory from low relative standard deviations (RSD) values of intraday studies (0.232 – 0.447) and inter-day studies (0.145 – 0.289) (Table 4).
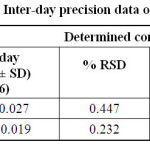 |
Table 4: Intraday and Inter-day precision data of the proposed method Click here to View table |
Ruggedness: No significant difference was observed between two analysts which was evident from the reproducible results. Hence, the proposed method could be considered as rugged (Table 5).
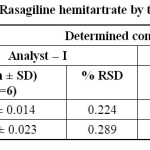 |
Table 5: Ruggedness data of Rasagiline hemitartrate by two analysts at different days Click here to View table |
Detection of LOD and LOQ: LOD and LOQ were found to be 0.033 and 0.10 µg mL–1 respectively and the results show that the proposed method was sensitive to detect and quantify24
Comparison of validation parameters
The results of validation parameters for the proposed method were within acceptable limits of ICH-2005 guidelines25. The correlation coefficient for the proposed method is 0.99937 indicating good linearity. %RSD in recovery of rasagiline hemitartrate by the proposed method (0.19-0.25) is lower compared to those values of visible spectrophotometric methods proposed by other workers4,26 for determination of its equivalent drug – rasagiline mesylate. LOD and LOQ values of the proposed method for rasagiline hemitartrate (0.033 and 0.1) are lower compared to those values of visible spectrophotometric methods proposed by other workers4,26 for determination of its equivalent drug – rasagiline mesylate. Hence, the proposed diazotization method for visible spectrophotometric determination of Rasagiline hemitartrate was proved to be better compared to red-ox / ion-pair complex formation methods proposed for the determination of its equivalent drug – rasagiline mesylate.
CONCLUSIONS
The proposed method for determination of rasagiline hemitartrate involves diazotization of sulphanilic acid under acidic conditions in presence of sodium nitrite, followed by its coupling with rasagiline in alkaline medium to form an yellow-colored chromogen. This method was tested and validated for various parameters according to ICH guidelines. The proposed method can be used for the routine quality control analysis of Rasagiline hemitartrate in bulk form.
REFERENCES
- Chen, J.J. and Ly, A.V., Am. J. Heal. Sys. Phar., 63(10): 915-928 (2006).
- Kathirvel, S., Satyanarayana, S.V. and Rao, G.D., Ch. Res. I., Article ID 273604, 1-6, doi:10.1155/2012/273604 (2012).
- Peskin, T.B., Rasagiline formulations of improved content uniformity, US Patent (2006).
- Chennaiah, M., Veeraiah, T., Singh, T.C. and Venkateshwarlu, G., J. Chi. Chem. Soc., 56(4): 926-929 (2011).
- Bhavinkumar, K.V., Raj. Gan. Uni. He. Sci., Quality Assurance, http://hdl.handle.net/123456789/5167 (2011).
- Raja, J.K., Jeyabaskaran, M. and Kumari, M.P., J. Phar. Res., 4(5): 1376-1377 (2011).
- Song, M., Wang, L., Zhao, H., Hang, T., Wen, A., Yang, L. and Jia, L., J. Ch. B, Anal. Tech. Biomed. L. Sci., 875(2): 515-521 (2008).
- Sudhir, M.S. and Nadh, R.V., Dr. Inv. Today., 5: 133 – 138 (2013).
- Sudhir, M.S. and Nadh, R.V., Accepted for Tr. J. Pharm. Res., Reference number of manuscript: MRN2083345.
- Mehta, R.S., Patel, D.M., Bhatt, K.K. and Shankar, M.B., Ind. J. Pharma. Sci. 67(4): 487-489 (2005).
- Raja, G.V., Triveni, Y., Divya, K., Lakshmi, K.N. and Gopal, G.V., Der Pharma Chemica., 1(2): 285-291 (2009).
- Krohn, K., John, M. and Demikhov, E.I., Rus. Chem. Bull., 50(7): 1248-1254 (2001).
- Zollinger, H., In Color Chemistry, Synthesis, Properties and Applications of Organic Dyes and Pigments, VCH, Weinheim, Germany, 85 (1987).
- Kirk-Othmer., In Encyclopedia of Chemical Technology, Dyes, Azo. Wiley, New York, USA, 349 (1982).
- Saunders, K.H. and Allen, R.L.M., Aromatic Diazo Compounds, 3. ed, Edward Arnold, London, UK, 1985.
- Watson, D., Cli. Chem., 7(6): 603-625 (1961).
- Furniss, B.S., Hannaford, A.J., Smith, P.W.G. and Tatchell, A.R., In Vogel’s Textbook of Practical Organic Chemistry, Aromatic compounds, Pearson Education limited, Longman group UK, 920 (1989).
- Zollinger, H., In Azo and Diazo Chemistry, Aliphatic and Aromatic Compounds, Inter Science Publishers, New York, USA, 125 (1961).
- Zollinger, H., In Color Chemistry, Syntheses, Properties, and Applications of Organic Dyes and Pigments, Azodyes and Pigments, Verlag Helvetica Chimica Acta, Postfach, Zurich, Switzerland, 167 (2003).
- Upadhyay, K., Rec. Res. Sci. Tech., 4(8): 89-91 (2012).
- Bladyga, J. and Bourne, J.R., Turbulent mixing and chemical reactions, Johon Wiley and Sons, Inc., New York, USA (1999).
- Revanasiddappa, H.D., Deepakumari, H.N., Mallegowda, S.M. and Vinay, K.B. Analele UniversităŃii din Bucuresti, 20(2): 189 – 196 (2011).
- Glaser, R., Wu, H. and Lewis, M., J. Am. Chem. Soc., 127(20): 7346–7358 (2005).
- Sethi, P.D., HPLC quantitative analysis of pharmaceutical formulations, CBS publications, 5. ed., India, 160 (2001).
- International Conference on Harmonization, (ICH), Validation of Analytical Procedures, Definition and Terminology, Q2 (R1) (2005).
- Rao. G.D., Kathirvel, S. and Satyanarayana, S.V., J. Ph. Res., 4(1): 61-62 (2011).

This work is licensed under a Creative Commons Attribution 4.0 International License.









