Corrosion behaviour of the 42CrMo4 Steel Nitrided by Plasma
Okba Belahssen*, Abdelouahed Chala , Said Benramache
Laboratoire de Physique des Couches Minces et Applications (LPCMA), university of Biskra. Algeria.
DOI : http://dx.doi.org/10.13005/ojc/290434
Article Received on :
Article Accepted on :
Article Published : 16 Jan 2014
This paper presents corrosion behaviour of alloy 42CrMo4 steel nitrided by plasma. Different samples were tested: untreated and plasma nitrided samples. The corrosion behaviour was evaluated by electrochemical techniques (potentiodynamic curves and electrochemical impedance spectroscopy). The corrosion tests were carried out in acid chloride solution 1M. The best corrosion protection was observed for samples nitrided.
KEYWORDS:steel 42CrMo4; nitriding; eis.
Download this article as:| Copy the following to cite this article: Belahssen O, Chala A, Benramache S. Corrosion behaviour of the 42CrMo4 Steel Nitrided by Plasma. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Belahssen O, Chala A, Benramache S. Corrosion behaviour of the 42CrMo4 Steel Nitrided by Plasma. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1744 |
Introduction
Plasma nitriding is a thermochemical process extensively applied in materials science and surface engineering due to its well-known potential for improving properties such as hardness, wear, and corrosion resistance of metallic parts [1]. This surface treatment technique consists of the implantation of nitrogen species at low energies into the steel substrate, and their subsequent diffusion into the bulk at temperatures above 300 °C. The interaction of nitrogen and steel constituents leads to the formation of different types of metallic nitrides, which form the so-called ‘‘nitride layer”. Starting from the solid surface, such a modified layer usually comprises an oxide layer, a compound zone, and a diffusion zone [2]. The resulting structure of these domains depends on several processing parameters such as the concentration of alloying elements, exposure time, substrate temperature, and gaseous mixture [3]. The presence of a nitride layer obviously changes the mechanisms of interaction between metallic materials and their surroundings, thus affecting their stability in aggressive environments [4]. The incorporation of nitrogen imparts better mechanical properties (friction and wear resistance), but the dissolution kinetics (corrosion resistance) remains closely related to the composition of the corrosive medium [5].
In this context, the 42CrMo4 steel is largely employed in industrial processes that take place in aggressive environments. Hard iron nitrides are originated during the plasma treatment owing to nitrogen diffusion in the near surface region at temperatures below the eutectic point (593 °C) [6]. Usually, two distinctive phases corresponding to the ε-Fe2-3N and γ’-Fe4N nitrides are obtained, whose high hardness improves the strength, friction and wear resistance [7]. Recent work has shown that the pitting corrosion resistance of steel can be significantly improved by nitride layers consisting of ε-Fe2-3N and γ’-Fe4N phases [19]. However, the effect of the nitride layer microstructure on the pitting corrosion behaviour of steel is still not fully understood. In this study, we address this question by analysing the influence of plasma processing at optimal parameters (temperature 500°C, exposure time 4h and gas mixture 20%H2, 80%N2) [9,10] on the corrosion behaviour of plasma-nitrided 42CrMo4 steel.
Experimental
42CrMo4 steel samples with nominal composition of 97.03 Fe, 0.40% C; 0.28% Si; 0.9% Mn; 1.09% Cr; 0.27% Mo; 0.015% P; and 0.018 % S (wt. %) were used in this study. The corrosion behaviour of nitrided alloy was evaluated by electrochemical impedance spectroscopy (EIS) measurements. Impedance data for 30 minutes of immersion were acquired by a potentiostat (AUTOLAB PGSTAT 30) and a frequency response analyzer system operating at open circuit potential in a frequency range from100 kHz to 10 Hz with a perturbation of ±10 mV. Experiments were conducted in a classical three electrodes cell. A saturated calomel reference electrode was used as reference electrode and a platinum (Pt) wire as counter electrode.
Results and discussion
Fig. 1 shows the potentiodynamic polarization curves in HCl solution for the samples untreated and nitrided. After nitriding, we can notice a slight shift of potential towards negative values from – 486.5 to – 470.05 mVSCE which result no passive layer at the bottom of the pores and its passivity requires applying an anodic potential. Despite its active state, it is however important to note that the nitride layer allows a significant reduction in the corrosion current density. The lower anodic current densities than for the untreated alloy, indicating an increase in the corrosion resistance, probably associated with the formation of the compound layer composed by Fe2-3N and Fe4N and/or to the enrichment of the metallic matrix in nitrogen.
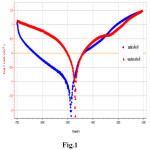 |
Fig. 1. Potentiodynamic polarization curves in HCl solution for untreated and nitrided alloy. Click here to View figure |
EIS experiments obtained at open circuit potential (OCP) in HCl solution were performed to characterize both the untreated and nitrided 42CrMo4 samples. Figs. 2a, 2b, 2c show the Nyquist and Bode plots of experimental data of the alloy. The nitrided sample shows a different behaviour due to the presence of nitrides with high chemical inertia. The high impedance values and phase angle of almost -60° at intermediate frequencies are indicative of a near capacitive response related to surface nitride. Moreover, a high impedance of nitrided sample confirms the low reactivity of the nitride alloy.
 |
Fig. 2. Nyquist(2a) and Bode(2b,2c) plots of experimental data of untreated and nitrided alloy. Click here to View figure |
The results for nitrided samples can be interpreted in terms of two time constants overlapped for a broad frequency range. The electrochemical behaviour for 42CrMo4 untreated was modelled by the equivalent circuit shown in figure 3 which is composed by circuit elements: Re, representing the solution ohmic resistance between the working and the reference electrodes, Q element, which represents a constant phase element (CPE) and describes a non-ideal capacitor when the phase angle is different from -90° [11]. CPE impedance is generally attributed to distributed surface reactivity, surface heterogeneity and roughness, to current and potential distribution related to the electrode geometry and porosity [9,12]. The circuit element RP, corresponds to the polarization resistance which is related to the charge transfer resistance at the metal/solution interface. W element is the Warburg impedance. The treated sample was modelled by the equivalent circuit shown in figure 4 which is
composed by elements: Re (the solution ohmic resistance), Q1and Q2 elements, which represent a CPE1 and CPE2 respectively, R1 resistance of the porous nitride layer, R2 the charge transfer resistance at the metal/nitide interface. The polarization resistance is RP = R1+ R2
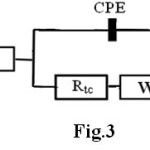 |
Fig. 3. Equivalent circuit for untreated sample. Click here to View figure |
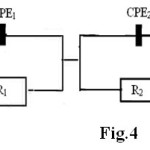 |
Fig. 4. Equivalent circuit for nitrided sample Click here to View figure |
The values of equivalent circuit elements resulting from simulation by the EC-Lab 10.02 software are shown in table 1 for untreated sample and in table 2 for nitrided one.
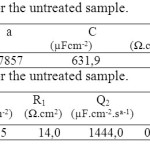 |
Table 1. Impedance parameters for the untreated sample.Table 2. Impedance parameters for the untreated sample. Click here to View table |
The lower Rp value 43.07 Ωcm2for untreated sample when compared to Rp value 73.6 Ωcm2 for treated one are attributed to a higher corrosion resistance for the nitrided alloy in this medium. The phase-angle profiles in the low frequency region in Fig. 4c confirm the predominant capacitive contribution on nitrided sample, maintaining high θ values for frequencies much lower than for the untreated alloy. All these features point to a higher corrosion resistance of the nitrided sample than of the untreated one.
Conclusion
In this work, it was shown that nitriding treatment applied to carbon steel enriched in chromium is adapted to the protection against corrosion of the 42CrMo4 steel. In optimal plasma treatment conditions, electrochemical tests showed that nitride layer provides good corrosion protection in HCl solution. The nitrided alloy shows a significant increase in corrosion behaviour.
References
- C. Blawert, A. Weisheit, B.L. Mordike, F.M. Knoop, Surf. Coat. Technol., 85, 15(1996).
- L.C. Gontijo, R. Machado, E.J. Miola et al., Surf. Coat. Technol., 183, 10(2004).
- M. Hudis, J. Appl. Phys., 44, 1489(1973).
- M.K. Lei, X.M. Zhu, J. Electrochem. Soc., 152, B291(2005).
- M.K. Lei, Z.L. Zhang, J. Vac. Sci. Technol., A15, 421(1997).
- T. Bell, Y. Sun, A. Suhadi, Vacuum, 59, 14(2000).
- T. Liapina, A. Leineweber, E.J. Mittemeijer, W. Kockelmann, Acta Mater., 52, 173(2004).
- L.L.G. da Silva, M. Ueda, R.Z. Nakazato, Surf. Coat. Technol., 201, 8291(2007).
- O. Belahssen, A. Darssouni, A. Chala, Ann. Chim. Sci. Mat., 33, 423(2008).
- O. Belahssen, A. Darssouni, A. Chala, Physical & Chemical News, 47, 84(2009).
- G.W. Walter, Corros. Sci., 26, 681(1986).
- M.F. Montemor, M.G.S. Ferreira, Electrochim. Acta, 52, 7486(2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.









