Cobalt salophen complex supported on magnetic nanoparticles as an efficient reusable catalyst for oxidation of benzylic alcohols
Mozhgan Afshari1*, Maryam Gorjizadeh1, Simin Nazari2 and Mohammad Naseh3
1Department of Chemistry, Shoushtar Branch, Islamic Azad University, Shoushtar, Iran 2Department of Chemistry, Sousangerd Branch, Islamic Azad University, Sousangerd, Iran 3Department of Chemistry, Dezful Branch, Islamic Azad University, Dezful, Iran
DOI : http://dx.doi.org/10.13005/ojc/290431
Article Received on :
Article Accepted on :
Article Published : 09 Jan 2014
A novel and general method has been developed for oxidation of benzylic alcohols using magnetic nanoparticles immobilized salophen Co(II) as an efficient and recyclable catalyst. The structural and magnetic properties of catalyst are identified by transmission electron microscopy (TEM), vibrating sample magnetometer (VSM) instruments. FT-IR, and XRD. Nanocatalyst can be easily recovered by a magnetic field and reused for subsequent reactions for at least 5 times with less deterioration in catalytic activity.
KEYWORDS:Cobalt ferrite;oxidation; Magnetic nanoparticles;Surface functionalization;Cobalt salophen complex
Download this article as:| Copy the following to cite this article: Afshari M, Gorjizadeh M, Nazari S, Naseh M. Cobalt salophen complex supported on magnetic nanoparticles as an efficient reusable catalyst for oxidation of benzylic alcohols. Orient J Chem 2013;29(4) |
| Copy the following to cite this URL: Afshari M, Gorjizadeh M, Nazari S, Naseh M. Cobalt salophen complex supported on magnetic nanoparticles as an efficient reusable catalyst for oxidation of benzylic alcohols. Orient J Chem 2013;29(4). Available from: http://www.orientjchem.org/?p=1518 |
INTRODUCTION
Transition metal Schiff base complexes have attracted much interest in the field of catalysis because of their reversible bonding oxygen ability and high activities in the oxidation reactions 1-7. However, these catalysts are generally homogeneous and are not easily recoverable after oxidation. Also, the work up procedure is not convenient and generates a lot of waste. One of the solutions of these problems is the immobilization of the catalyst on the solid support. Several insoluble solid materials either organic polymers or inorganic porous have been frequently applied as heterogeneous catalytic support for these complexes 8-10. Inorganic matrices show some advantages over organic supports such as high thermal, chemical and mechanical stability. However, due to the diffusion of substrates and products through the pores of the support materials, a substantial decrease in reaction rate is frequently observed compared to the homogeneous system 11-14. Thus, it is worthwhile to consider new materials as support for these homogenous catalysts.
Nowadays, magnetic nanoparticles (MNPs) have attracted increasing interest as promising alternatives for the immobilization of homogeneous catalysts because of their unique properties including a large surface-to-volume ratio, magnetism, low toxicity, biocompatibility and their potential applications in various fields 15-20.
In this study, we wish to present a novel method for the immobilization of a Co (II) salophen complex on imidazole functionalized silica coated cobalt ferrite nanoparticles (Co (salophen)-imid-Si@Si-MNPs). The catalytic activity and reusability of this composite in the oxidation of benzylic alcohols was investigated.
EXPERIMENTAL
GENERAL
Chemical materials were purchased from the Merck Chemical Company in high purity. Cobalt ferrite MNPs were prepared using the procedure reported by Maaz et al. 21. Silica coated CoFe2O4 nanoparticles (Si-MNPs) were made by using sol-gel method 22. X-ray diffraction (XRD) patterns were recorded with a Philips X-ray diffractometer (Model PW1840). FT-IR spectra were obtained using BOMEM MB-Series 1998 FT-IR spectrometer. Magnetic properties of all nanoparticles were measured with a vibrating sample magnetometer (VSM, Meghnatis Daghigh Kavir Company, Iran) at room temperature. NMR spectra were recorded in CDCl3 on a Bruker Advanced DPX 400 MHz spectrometer.
SYNTHESIS OF Co (salophen)-imid-Si@Si-MNPs
A schematic representation for the synthesis of Co (II) salophen complex anchored on imidazole functionalized silica coated cobalt ferrite nanoparticles is shown in Scheme 1. In the first step, imidazole functionalized silica coated magnetic nanoparticles (imid-Si@ Si-MNPs) were achieved by using the previously reported method 23.
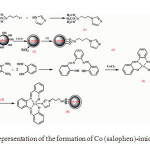 |
Scheme 1. Schematic representation of the formation of Co (salophen)-imid-Si @ Si-MNPs catalyst Click here to View figure |
Co (II) complexes of Schiff base ligands have been synthesized by the reaction of CoCl2 in ethanolic solutions with the Schiff bases obtained by the condensation of 1, 2 -phenylenediamine (2 mmol, 0.216 g) with salicylaldehyde (4 mmol, 0.43 ml). Then Co (II) salophen complex was dissolved to 1, 2 dichloroethane and imid -Si@ Si-MNPs (0.75 g) were dispersed to it and the whole mixture was refluxed for 2 days. After that, the expected final product, Co(salophen)-imid-Si @ Si-MNPs , was separated by magnetic decantation and washed with 1,2 dichloroethane, methanol and chloroform until the washings were colorless and left to dry in a desiccator.
CATALYTIC STUDIES
In a typical run, a 25 mL flask was charged with catalyst (100 mg), acetonitrile (5 mL), alkene (5 mmol), and H2O2 (3 mmol). This mixture was heated in an oil bath at 70 ˚C and the progress of the reaction was monitored by TLC. At the end of the reaction, the catalyst was simply collected using a magnetic bar and the reaction mixture was then transferred out of the flask. The decanted solution was purified on a silica-gel plate or a silica-gel column to obtain the pure product. The structures of the products were established on the basis of their spectral analysis (IR, 1H NMR).
RESULTS AND DISCUSION
CHARACTERIZATION OF Co (salophen)-imid-Si@Si-MNPs
The catalyst was synthesized by a procedure that shown in Scheme 1, and characterized by various techniques.
The FT-IR spectrum of heterogenised cobalt salophen shows the expected principal bands for the free schiff base complex. The C-H deformation band is visible at 1470 cm-1 and the band at 1611 cm-1 was assigned to –C=N stretching vibration of the immine group of the ligand that were not in the imidazole functionalized silica coated cobalt ferrite. The other bands at 589cm-1, 802 cm-1 and 1088 cm-1 ascribed to Fe-O stretching absorption, asymmetrical and symmetrical vibrations of the Si-O-Si bond respectively (see Fig. 1) 24,25.
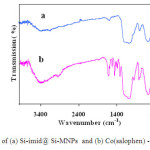 |
Fig.1. FT-IR spectra of (a) Si-imid@ Si-MNPs and (b) Co(salophen) -imid-Si @ Si-MNPs Click here to View figure |
XRD measurements of Co(salophen)-imid-Si @ Si-MNPs exhibit diffraction peaks corresponding to the cubic reverse spinel structure of CoFe2O4 (JCPDS PDF #221086), while the silica layered systems show an additional broad band at 2θ=20–30 (Fig. 2b and 2c) for the amorphous silica. The average crystallite size of the Co(salophen)-imid-Si @ Si-MNPs was calculated to be ~28 nm by Debye-Scherer formula for the (311) reflection.
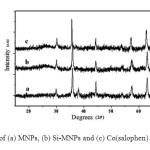 |
Fig. 2. XRD pattern of (a) MNPs, (b) Si-MNPs and (c) Co(salophen) -imid-Si @ Si-MNPs Click here to View figure |
The TEM micrograph of the nanoparticles is shown in Fig.3. TEM image indicated that most of the prepared nanoparticles are spherical shaped and have size less than 30 nm which show a close agreement with the values calculated by XRD analysis. Interestingly, the magnetic core is visible as a dark spot inside the bright spherical SiO2 thin shell in the TEM image of Co(salophen)-imid-Si @ Si-MNPs sample.
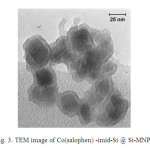 |
Fig. 3. TEM image of Co(salophen) -imid-Si @ Si-MNPs Click here to View figure |
The magnetic properties of the silica coated cobalt ferrite (Si-MNPs)and Co (salophen)-imid-Si @ Si-MNPs were studied by a vibrating sample magnetometer at 300 K. The saturation magnetization (Ms), remanence magnetization (Mr) and coercivity (Hc) values of these compounds are given in Table 1. As clearly shown, the saturation magnetization of coated sample is slightly less than that of uncoated sample, whereas the coercivity of coated sample is larger than that of uncoated one. This is probably due to the formation of non-crystalline silica on the surface of CoFe2O4 nanoparticle as well as the restriction of domain wall motion by the magnetic dilution effect of inert silica.
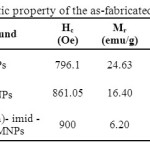 |
Table1. Magnetic property of the as-fabricated nanoparticles. Click here to View table |
As can be seen from thermogravimetric analysis (TGA) the catalyst shows a good thermal stability (~176ºC), with a weight loss at temperature between 173-753ºC, which is characteristic of chemisorbed materials and confirmed that the organic group is covalently bound on the surface of Si-MNPs (Figure 4). On the basis of the TGA an organic group loading at ca. 0.44 mmol g−1 is obtained. Moreover, the amount of excess cobalt, in addition of Fe/Co ratio in cobalt ferrite of the catalyst, determined by ICP-AES, was 0.3 mmol g−1. This is another proof for the fact that Co (II) salophen complex was covalently supported on imidazole functionalized silica coated CoFe2O4 nanopaticles.
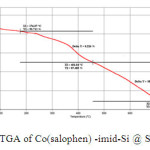 |
Fig. 4. TGA of Co(salophen) -imid-Si @ Si-MNPs Click here to View figure |
OXIDATION OF ALCOHOLS
In order to optimize the reaction conditions for the oxidation of benzylic alcohols various experiments were carried out at 70 ˚C using 1-phenylethanol in the presence of heterogenised cobalt salophen as catalyst and H2O2 as an oxidant.
To optimize the catalyst requirement, the amount of catalyst was changed between 25 and 200 mg per 5 mmol 1-phenylethanol alcohol. Increase in the yield of acetophenone was observed when the amount of catalyst was increased from 25 to 100 mg but the yield remained almost same with further increment of catalyst amount up to 200 mg. So an optimum of 100 mg catalyst in the reaction mixture is ideal for achieving the best yield. In addition, in the absence of catalyst, the reaction was found to be very slow and was not complete even after 8 h.
We have also accomplished the oxidation of 1-phenylethanol in the different solvents such as n-hexane, acetone, toluene, cyclohexan, chloroform, dichloromethane and also acetonitrile. Among the solvents examined, we found acetonitrile to be the best for this protocol due to high yield and good dispersibility of the catalyst in this media. Replacing H2O2 by t-BuOOH, NaIO4 or H2O2/ Urea (UHP) cause to decrease the obtained total oxidation yields, so H2O2 was selected as a best source of oxygen for this system.
At optimum conditions we observed that when a heterogeneous mixture of this alcohol and Co(salophen) -imid-Si @ Si-MNPs was heated at 70 ˚C in acetonitrile, acetophenone was formed as the only product in 94% yield after 1.5 h.
The obtained preliminary results for the oxidation of 1-phenylethanol were encouraging and to extend the scope of this heterogeneous catalytic system, the oxidation of further alcohols was carried out under the optimized conditions. The given results in Table 2 show that this procedure was clean and all the alcohols were converted into the corresponding carbonyl compounds in moderate to excellent yields without over-oxidation to carboxylic acids.
 |
Table2. Oxidation of alcohols Co (salophen)-imid-Si@ Si-MNPs catalyst a Click here to View table |
interesting electronic effect seemed to exist for these benzylic systems. For example, p-nitrobenzyl alcohol (Table 2, entry 9) required a longer reaction time and gave a decreased yield compared to more electron-rich benzylic substrates. For investigating the chemoselectivity, a mixture of 1-phenylethanol and 2-phenylethanol was subjected under optimized condition in the presence of Co(salophen) -imid-Si @ Si-MNPs. Result indicated that the former was oxidized to acetophenone in 94% yield while 2-phenylethanol was recovered completely. Furthermore, this reaction is highly selective for vicinal diols in oxidizing only the secondary hydroxy group α to the benzene ring (Table 2, entries 15). Therefore, it is a method of choice for the oxidation of benzylic OH in the presence of non-benzylic OH.
To evaluate the stability and level of reusability of the catalyst, we conducted experiments of oxidiation of 1-phenylethanol using the recycled Co (salophen)-imid-Si@ Si-MNPs catalyst. After the completion of the first reaction, the catalyst was concentrated on the sidewall of the reaction vessel using an external magnet and the solution was removed by decantation and the left used catalyst was washed with CH3CN. A new reaction was then conducted with fresh reactants under similar conditions. It was found that the developed catalyst could be used at least five times without any change in reactivity (Table 2, entries 16). Compared with the homogeneous and heterogeneous same catalysts our novel catalyst has shown much more superiority. The observed high activity of the catalyst can be attributed to the nanosized character of the support which increases surface area for more interaction with substrates26-28.
CONCLUSION
In summary the cobalt salophen complex has been supported on the surface-modified CoFe2O4 magnetic. The synthesized catalyst was confirmed by XRD, FT-IR, TGA, TEM, ICP-AES, and VSM techniques. This nanocatalyst was employed as an efficient catalyst for the oxidation of some benzylic alcohols with hydrogen peroxide. Moreover, the immobilized catalyst could be easily recovered by simple magnetic decantation and reused at least five times without significant loss of activity.
ACKNOLEDGMENT
Authors are thankful to Research Council of Shoushtar Branch, Islamic Azad University, Shoushtar, Iran, for providing financial support for this project.
REFRENCES
- E.N. Jacobsen, W. Zhang, M.L. Guler, J. Am. Chem. Soc. 113, 6703 (1991).
- W. Zhang, J.L. Loebach, S.R. Wilson, E.N. Jacobsen, J. Am. Chem. Soc. 112, 2801 (1990)
- M. Salavati-Niasari, M. Shakouri-Arani, F. Davar, Microporous Mesoporous Mater. 116, 77 (2008).
- M. Salavati-Niasari, Microporous Mesoporous Mater. 92,173 (2006).
- M. Salavati-Niasari, Microporous Mesoporous Mater. 95, 248 (2006).
- K.J. Balkus Jr., A.K. Khanmamedova, K.M. Dixon, F. Bedioui, Appl. Catal. A Gen. 143, 159(1996).
- M.R. Maurya, S.J.J. Titinchi, S. Chand, J. Mol. Catal. A Chem. 214,257 (2004).
- S. Bhunia, S. Koner, Polyhedron, 30, 1857, (2011)
- B. Qi, X. H. Lu, S.Y. Fang, J. Lei, Y.L. Dong, D. Zhou, Q.H. Xia, J. Mol. Catal. A 334, 44, (2011)
- G. Grivani, S. Tangestaninejad, A.R. Halili, Inorg. Chem. Commun. 10, 914, (2007)
- P. Ferreira, I.S. Goncalves, F. E. Kuhn, A.D. Lopes, M.A. Martins, M. Pillinger, A. Pina, J. Rocha, C.C. Romao, A.M. Santos, T.M. Santos, A.A. Valente, Eur. J. Inorg. Chem. 2263, (2000)
- C.D. Nunes, A. A. Valente, M. Pillinger, A.C. Fernandes, C.C. Romao, I. Rocha, I.S. Goncalves, J. Mater. Chem. 12, 1735, ( 2002)
- M. Jia, A. Seifert, W.R. Thiel, Chem. Mater. 15, 2174, (2003)
- A. Sakthivel, J. Zhao, G.R. Sieber, M. Hanzlik, A.S.T. Chiang, F.E. Kuhn, Appl. Catal. A. 281, 267, (2005)
- A. Jutand, S. Negri, A. Principaud, Eur. J. Org. Chem. 631(2005).
- S. Cacchi, E. Morera, G. Ortar, Synthesis, 320 (1986).
- M. Alami, F. Ferri, G. Linstrumelle, Tetrahedron Lett. 34, 6403(1993).
- M. Sevilla, T. Valdés-Solis, P. Tartaj, A.B. Fuertes, J. Colloid Interf. Sci. 340, 230(2009).
- M. Mahmoudi, S. Sant, B. Wang, S. Laurent, T. Sen, Adv. Drug Deliver. Rev. 63,24(2011).
- V. Polshettiwar, R.S. Varma, Green Chem. 12, 743 (2010).
- K. Maaz, A. Mumtaz, S.K. Hasanain, A. Ceylan, J. Magn. Magn. Mater. 308, 289, (2007)
- Y.H. Deng, C.C. Wang, J.H. Hu, W.L. Yang, S.K. Fu, Colloids Surf. A 262, 87, (2005)
- M. Kooti, M. Afshari, Mater. Res. Bull. 47, 3473, (2012)
- M. Ma, Y. Zhang, W. Yu, H.Y. Shen, H. Zhang, N. Gu, Colloids Surf. A 212, 219, (2003)
- H. Ono, T. Katsumata, Appl. Phys. Lett.78, 1832, (2001)
- F. Rajabi , B. Karimi, J. Mol. Catal. A Chem. 232,95 (2005).
- H. Golchibian, S. E. Babaei, Chin. J. Catal., 31,615 (2010).
- N. Gunasekaran, P. Jerome, S. W. Ng, E.R.T. Tiekink, R. Karvembu, J. Mol. Catal. A Chem. 353,156 (2012).

This work is licensed under a Creative Commons Attribution 4.0 International License.









