Synthsis, Specrtoscopic, Electrochemical and Antibacterial Activities of Mono- and Homobi-Nuclear Of Ru (Ii) Complexes with a Triazolate Bridging Ligands
Mohamed A. EL-GAHAMI*,1,3, Hssan M. ALBISHRI1
1Department of Chemistry, Faculty of Science, King Abdulaziz, North Jeddah, KSA
2Department of Chemistry, Faculty of Science, King Abdulaziz, P.O.Box 80203, Jeddah, KSA
3Chemistry Department, Faculty of Science, Assiut university, Assiut ,EGYPT
DOI : http://dx.doi.org/10.13005/ojc/290308
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
Mono- and homobi- nuclear Ru (II) complexes were prepared by the reaction of [Ru (bipy) 2Cl2] H2O with 4-amino-3,5-DI (2-byridyl) -4H-1,2,4-triazole (adapt); ( 4-byridyl) -3,5-DI (2-byridyl) -4H-1,2,4-triazole (Pydpt), and 4-( 4-methylphenyl) -3,5-DI (2-byridyl) -4H-1,2,4-triazole (mpdpt). The products were characterized by elemental analysis, IR, electronic and 1H NMR spectral however molar conductance and their Redox chemistry has been investigated using cyclic –voltmeter have been studied. An octahedral structure has been tentatively proposed for the new complexes. All the complexes exhibit reversible, metal-based oxidation processes and reversible, bipy-based reduction process in the potential window investigated. . The metal complexes are more active than the parent ligands and exhibit mild to moderate antibacterial activity.
KEYWORDS:Synthesis Ru (II) complexes;triazolate bridge ligands;spectroscopy;electrochemical and antibacterial activity
Download this article as:| Copy the following to cite this article: El-Gahami M. A, Albishri H. M. Synthsis, Specrtoscopic, Electrochemical and Antibacterial Activities of Mono- and Homobi-Nuclear Of Ru (Ii) Complexes with a Triazolate Bridging Ligands. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290308 |
| Copy the following to cite this URL: El-Gahami M. A, Albishri H. M. Synthsis, Specrtoscopic, Electrochemical and Antibacterial Activities of Mono- and Homobi-Nuclear Of Ru (Ii) Complexes with a Triazolate Bridging Ligands. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=272 |
INTROUDUCTION
Substituted 1,2,4-triazoles has been actively studied as bridging ligands between metal (II) ions coordinating through their N1 and N2 atoms, since their complexes have interesting structures and specific magnetic properties.1,2 Interesting to note that some complexes containing substituted 1,2,4-triazole ligands have the spin-crossover properties, which could be used as molecular-based memory devices, displays and optical switches. 3,4
The substituted 1,2,4-triazole has been also of magnetochmical interest because it is able to act as a bridge between metal centers thus mediating exchange coupling. 5-7 Ru (II) polypyridyl complexes have been reported and exhibit interesting spectroscopic, photophysical, photochemical , coupled to catalytic and electrochemical properties due to their application, in the construction of Suparmolecular devices. 8-13
The general approach, utilized in electron transfer studies has been to bind the electron acceptor/donor to the ruthenium polypyridyl center via the polypyridyl Ligands. 14-17 In the present study, we describe the synthesis and spectroscopic (UV, IR, 1HNMR) studies of new mono-and homobi-nuclear Ru (II) complexes containing triazolate bridge ligands. Their redox chemistry has been studied by cyclic voltmeter. The structures of the ligands used in the present studies are given in Scheme 1.
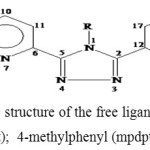 |
Scheme 1. The structure of the free ligands Where R=NH2 (adpt); 4-methylphenyl (mpdpt); 4-pyridyl (pydpt) Click here to View figure |
EXPERMENTINAL
4-amino-3, 5-di (2-byridyl)-4H-1,2,4-triazole was obtained from Aldrich . Commercially available RuCl33H2O was used as supplied. All the reagents used were of Analar or chemically pure grade solvents were purified and dried. Electronic spectra were run on a Perkin Elmer UV/Vis spectrophotometer lambda 40 using 1-cm matched silica cells. IR spectra were obtained in KBr discs using 470 Shimadzu infrared spectrophotometer (4000-200 cm-1). Conductivity measurements were carried out using CDM216 Meter lab conductivity meter in DMF solutions at 10-3M concentrations at room temperature (~25 0C). Elemental analysis of the solid complexes was performed in an Elementary Analyzer system GmbH Vairo El. 1H NMR spectra were recorded on a Broker 300 MHZ spectrophotometer. The chemical shifts are reported using the residual solvent signal as an indirect reference to TMS: DMSO-d6 2.05 ppm (1H).
The electrochemical behavior as studied in acetonitrile solution at room temperature by cyclic Voltammetry and differential pulse Voltammetry by using a platinum electrode and saturated silver chloride reference electrode with 0.1mmol L-1 TBAP as supporting electrolyte. . Cyclic voltammetry (CV) was conducted using an EDTECP 133 potential/galvanostat together with a JJPL3 X-Y recorder. All samples were purged with nitrogen prior to measurement.
Differential pulse Voltammetry (DPV) was carried out on an EG&G model 264A polar graphic analyzer together with an EG&G G2000 X-Y recorder.
Preparations Cis-[(bipy)2RuCl2]2H2O
The following modification of the preparation of this complex developed by Weaver 18 was utilized to give good yields of the complex.
Commercial RuCl3.3H2O (2.06 g/cm3, 10 mmol/L), bipyridine (3. 11 g/cm3, 20 mmol/L) and LiCl (2.08 g/cm3, 1mmol/L), were heated at reflux in reagent grade dimethyl formamide (20 mL) for 8h. The reaction was stirred magnetically throughout this period. After the reaction mixture was cooled to room temperature, 100 mL of reagent grade acetone was added and the resultant solution cooled at OC over night. Filtering yielded a red to red-violet solution and dark green black microcrystalline product. The solid washed with portions of water followed by portions of diethyl ether, and then it was dried by suction.
Preparation of 4-(4-Pyridyl) 3, 5- (2-pyridyl)-4H-1, 2, 4- triazole (pydpt)
Pydpt was prepared as previously described. 19 The reaction of N-(-4 pyridyl) pyridine-2-thiocarboxamide (4.03 g/cm3, 20 mmol/L) and pyridine-2-carbohydrazide (3.29 g/cm3, 20mmol/L) in 1-butanol (80 mL) was refluxed for 24 h. On cooling, the product crystallized from the orange reaction mixture. It was filtered off, washed with ethanol and dried in vacuum to give 60 % of analytical pure pydpt as fine colorless needles mp. 265 0C. C17H12N6 (300.32): calced. C 67.99, N 27.98, H 4.03; Found: C 67.78, H 4.19, N 27.70. 1H NMR =7.20 – 7.22 (m, 2H, 3′ and 5′-PyH), 7.24 (ddd, 3J4.5= 7.7, 3J5.6 = 4.8, 4J3.5 = 1.2 HZ, 2H, 2×5′-pyH), 7.24 (d, 3J3.4 = 3J4.5 = 7.7, 4J4.5 = 1.8 Hz,2H, 2×4-pyH), 8.21 (ddd, 3J3.4 = 7.7, 4J3.5 = 1.2, 5J3.5= 0.9 Hz, 2H, 2×3-pyH), 8.28 (ddd 3J5.6= 4.8, 4J4.6 = 1.8, 5J3.6 = 0.9 Hz, 2H, 2x6py H), 8.62-8.64 (m, 2H, – and – pyH) ppm.
Preparation of 4-(4-methylphenyl)-3,5-di(2-pyridyl)-4H-1,2,4-triazole( pmdpt)
Pmdpt was prepared as previously described 13. The reaction of crude N-(-4methylphenyl) pyridine-2-carboxyimidothioate (5.13 g/cm3, 20 mmol/L) and pyridine-2- carbohydrazide (3.29 g/cm3, 25 mmol/L) in 1-butanol (50 mL), following the procedure described above for the preparation of pydpt. mp. 205 0C, C19H15N5 (313.36); calced. C72.84, H 4.80, N 22.47; found C 72.65, H 4.6, N 22.60. 1H NMR = 2.37 (3H, CH3), 7.09 – 7.14 (m, 4H, 2-,3-, 5- and 6- phMeH) 7.22 (ddd, 3J4,5 = 7.7, 3J5.6 = 4.8, 4J3.5 = 1.2 Hz, 2H, 2×5-pyH), 7.73 (dt, 3J3,4=7.7, 4J3.5 = 1.2, 5J3. 6= 0.9 Hz, 2H,
2×6. pytH) ppm.
Preparation of mono-nuclear Ru (II) complexes
A solution of [Ru (bipy)2 Cl2]2H2O (0.2 g/cm3, 1mmol/L) in ethanol (20 mL) was boiled for 10 min. and filtered into a solution of adpt (3.0 mg/cm3) or pydpt (0.5 g/cm3, 1 mmol/L) or pmdpt (0.35 g/cm3, 1 mmol/L) in ethanol (20 mL), the mixture was reflux for 5 h., and allowed to cool slowly to room temperature. On standing for several hours product crystals precipitated. The products were filtration and separated.
Preparation of homobi-nuclear Ru (II) complexes
This was prepared as above using [Ru (bipy)2 Cl2]2H2O (0.4 g/cm3, 1 mmol/L) in ethanol (20 mL) was boiled and added to ethanol solutions containing 0.3 g/cm3, of adpt ligand or 0.5 g/cm3 of pydpt or 0.35 g/cm3 of pmdpt the mixture was reflux for 8 h. and cooling. The complexes precipitated and filtrations on cooling.
Antibacterial activity
The antimicrobial activity of the complexes against different strains of bacteria was determined by the copulate method and activity was expressed in terms of diameters in mm zones of inhibition. In column was reported by washing a fresh 5 mL medium slant of test organism with 5mL sterile water and further dilution the 1ml washing to 10 mL . The suspension was added to 15 mL medium at a temperature 45-50 0 C and plats were prepared. Holes were into the agar plates with a sterile borer and filled with the drug. The plates were incubated at 35 0 C for 24 h. The results were compounded with that of standard chloramphenicol.
RESULTS AND DISCUSSION
Elemental analyses and molar conductance
The reaction of [Ru (bipy) 2Cl2]2H2O with L (where L = adapt, Pydpt, and pmdpt) yields the corresponding [Ru (bipy) 2L] Cl2. nH2O and [Ru2 (bipy) 4L] Cl4. nH2O, complexes I and II (Scheme 2), 1H NMR, IR and Microanalysis data support this formulation for I and II. Analytical results, color, and molar conductivity values for the prepared complexes are given in Table 1. The ligands used in this study in flexidentate where it can act as neutral bi- or tetra- dentate ligands. The results of the elemental analyses are constituent with 1:1 or 2:1 metal ion to ligand.
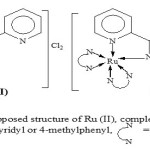 |
Scheme 2: Proposed structure of Ru (II), Complexes where R = NH2 or 4-byridyl or 4-methylphenyl N-N=2–bipyridine Click here to View figure |
The prepared complexes are stable at room temperature non-hygroscopic and insoluble in water and common organic solvents but dissolved in acetonitrile, DMF and DMSO.
The measured molar conductance values of the complexes in DMF (10-3 molL-1 ) solutions were measured at room temperature (~25 0C) and the results are listed in Table 1. The values show that mono- and homobi-nuclear complexes which are electrolytes 20. These values suggest the existence of the complexes as 1:2 and 1:4 species compared to other known Ru (II) complexes. 21,22 Complex II revealed that the two Ru (II) ion are mono triazole-bridged. Each Ru (II) is coordinated by one byridine and triazole nitrogen as well as a two bipyridyl molecule, completing the distorted octahedral coordination sphere about the metal centers. Based on spectroscopic and analytical data both forms have the formula for mono-and homobi-nuclear Ru (II) complexes given above and are most likely examples of linkage isomerism in which the adept ligand coordinates, in the A and B bidentate configurations (Scheme 3). The IR and H1 NMR data given after confirmed the adpt ligand coordinates in the B configurations
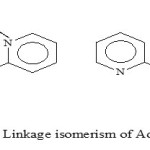 |
Scheme 3: Linkage isomerism of Adpt Ligand Click here to View figure |
Infrared spectra
The prominent IR spectral bands are present in Table 2, and reveal that ligands moieties are coordinated to the metal ion. The observed shift of the ligand bands in the corresponding complexes was taken as evidence for the coordination of the ligands to Ru(II) ion.
The NH2 stretching vibration at 3300 cm-1 for the free adpt ligand moiety is found to be the same position in the spectra of the complexes. This indicates that this group is not coordinated to the Ru (II) ion23 this confirmed the adpt ligand coordinated to Ru (II) ion in the B configuration. The main IR spectral variations of complexes compared to the free ligands are found in the 420-440 cm-1 region (out-of-plane – ring-deformation) and at 620-635 cm-1 (in – plane – ring – deformation)
Respectively 24,25 where the absorption of the pyridine ring of the ligands occurs. These vibrations are found to be positively shifted, suggesting coordination of the pyridine atoms N13 and N7 to the Ru (II) ion. However ring vibrations in the higher frequency region (1560-1580 cm-1) have not been affected appreciably.
The spectrum of free ligands shows a strong band in the range (1585-1625 cm-1), attributable to the pyridine ring vibrations. Upon pyridine coordination to a metal the higher bands are shifted by about +15 waves-numbers. 26 So in the spectrum of the mononuclear complexes a band at 1605 cm-1 and the two bands at 1590 and 1587 cm-1 can be assigned to one coordinated and one uncoordinated pyridine ring respectively. This means that in these mononuclear complexes each ligand uses one pyridine nitrogen and one triazole nitrogen of chelate binding. A negative shift (20-35 cm-1) in the (C=N) vibration of the triazole ring suggests 27 coordination via its N3 atom. The IR data show that this the case for both pyridine rings of the ligands in he binuclear complexes as conclude from the 1:2 (L:M) compositions this means that both pyridine nitrogen together with two of the triazole nitrogen N3 and N4 coordinated to the Ru(II) ions. This confirmed that in binuclear Ru (II) complexes each Ligand use triazole nucleus as bridge Ligand.
The IR spectra of the Ru (II) complexes showed broad bands in the range 3400-3450 cm-1 which can be assigned to (OH) of no- coordinated H2O molecules associated with the complexes which are confirmed by elemental analysis.
Electronic spectra
Absorption spectra of the Ru (II) complexes are studied in DMF solution. The concentration of all complexes is 1×10-4 molL-1 and the data summarized in Table 2. Assignments of the absorption of Ru (II) complexes were made on the basis of well- documented optical transitions of analogous Ru (II) polypyridine complexes. 28
The new Ru (II) triazolate complexes are diamagnetic, indicating the presence of the Ru+2 oxidation state. The ground state of Ru (II) ion (configuration) is 1A2g. The excited state, corresponding to configuring, is T1g, 3T2g, 1T1g and 1T2g in the order of increasing energy. Hence, four bands are possible corresponding to the 1A1g →3T1g, 1A1g→3T2g, 1A1g→1T1g and 1A1g→1T2g transitions.
In the electronic spectra of all complexes, we observed two bands in the 500-280 nm region. These at the longer wavelength (500-445 nm) have been assigned to the spin allowed 1A1g →1T1g transition based one molar extinction coefficients in the range 2800-3700 dm3 mol-1cm-1. 29,30 This band has absorption maxima of the complexes are blue shifted compared with that of Ru (bipy) 32+, 31 suggesting that the donor properties of the ligands are weaker than that of 2,2– -bipyridine. The extinction coefficients this band Ru (II) complexes are larger than those of Ru (II) complexes containing 2,2– -bipyridine, but smaller than those of Ru (II) complexes containing 2,2, –6–, 2= -tripyridine. 32
The other high intensity bands at 300-280 nm region (19000-2400 dm3 mol-1 cm-1) has been assigned to the charge transfer transition arising from the excitation of an electron from the metal T2g level to the unfilled MO’s derived from the level of the ligands, in accordance with the assignments made for other similar octahedral Ru(II) complexes. 33-34 The position of the lower (bipy) d (Ru) CT absorption in the cationic triazole complexes is very close to the observed in the corresponding pyridine complexes. This suggests that in this type of complex 1, 2, 4-triazole have similar -acceptor capacities to pyrazole but are weaker -acceptors than pyridine complex, the slight variation in band position with the change of the substituent on the 4-position suggests that these groups influence the acceptor properties of the traceable to some extent.
1H NMR spectroscopy
Previously an elaborate discussion has been given on the H1 NMR spectra because of the structural similarities between the mono -and homobi -nuclear compounds. As shown in Table 4 .The 1H NMR spectra are indeed very alike. The doublet at 5.57-6.77 ppm is noteworthy as it has previously been shown, 35 that this proton has a very short distance to an adjacent bipy ligand, thus yielding a strong up field shift. In contrast to what was reported before, the signals at around 7.1 ppm are due to H6 protons of the second pyridine rings of adpt and not due to the H3 protons. The chemical shifts of the bipyridyl ligands are essentially the same as reported Before. 36-38
The H6 proton of the pyridine ring of the pyridyl triazole ligand has been shifted about 1 ppm up field, which is caused by the anisotropic magnetic interaction of H6 within adjacent pyridine
ring. 39 The other protons are also shifted somewhat, because of the influence of the metal ion. In configuration B involves coordination through the pyridyl and triazole groups, while in configuration A coordination occurs through the pyridyl and amino groups. The major difference in the 1H NMR spectra for the two forms of B is the no chemical shifts of the amino protons. The amino protons as a singlet at 7.51 ppm in the free ligand and its complexes.
The 1H NMR spectrum of mpdpt complexes shows triplets at 2.85 ppm, corresponding adjacent to the (6H, CH3-ph). Furthermore, a broad signal appearing Ca. 1.84 ppm may be assigned primary amine protons (6H, NH2) of the adpt moiety.
Chemical shifts of the pyridine ring protons coordinated to the metal atom are noticing, different from those observed in a non-coordinated to pyridine ring of the ligands in mononuclear complexes. The proton resonances of the coordinated pyridine ring occurred 40 at 8.54, 8.24, 7.49 and 8.56 ppm while the resonances of non-coordinated ring protons appeared in 7.24, 8.10, 7.31 and 8.28 ppm.
Electrochemical measurements
Redox properties of the mono-and bi-nuclear complexes are present in Table 5. The complexes measured in CH3CN solution. By comparison with the well-known oxidation behavior of Ru (II) complexes of polypyridine ligands. 41,42The difference in peak positions (Ep=Epox-Epred) for the oxidation waves was 60-80 mV, indicating reversible electron-transfer processes. The difference in oxidation potentials between the two oxidation waves of the deprotonated binuclear compound is 0.32 eV. This indicates either that the chemical environments of the Ru-(bipy) 2 moieties are different due to the different coordination modes or that electrostatic interactions and/or electron delocalization are important in binuclear compound. Electrochemical studies of the Ru (II) complexes show one Ru(II) – centered oxidation and three ligands centered reductions however agree with literature results.43
Mono- and Homobi-nuclear Ru (II) centered reversible oxidation couple at 1.13-1.30V and 1.01-1.05V respectively. The potential of binuclear complexes is slightly more negative and mono-nuclear complexes are slightly more positive than that of Ru (bipy) 3, 44 which indicates that the ligands are a stranger π- acceptor than bipy but a weaker π- acceptor than Ru (II) complexes. 45
The oxidation potentials of mononuclear complexes are the same. The oxidation potentials of the Ru (II) complexes have been scheming. This suggests that the mode of coordination of the triazole does not affect the redox properties much. Therefore, the large difference in oxidation potentials of 0.32 V is most likely caused by electronic interactions between the two metal centers. This is further supported by the fact that the oxidation potential of the mononuclear complexes is slightly different from the oxidation potential in the binuclear complexes. In general binuclear compounds with small electron delocalization and resonance stabilization effects the first oxidation potential is expected to be similar to that observed for the mononuclear complexes. In addition of the Ru2+/3+ wave two more redox couples are found at about – 1.45V and -1.7Vs.
SCE is observed which are most likely due to a stepwise reduction of the bipyridyl legends. Similar redox couples have been reported in other Ru (II) (bipy) 2 complexes. All the redox couples observed appeared to be reversible.46,47
In the reduction processes of the complexes, the first reduction process is irreversible due to adsorption of reduced species onto the surface of the electrode. The first reduction process of Ru(II) complexes in the range (-1.42) – (-1.49) V is consistent with the addition of electrons to the localized on ligands. The second quasi- reversible reduction process in the range (-1.56) – (-1.77) V is located on one of the two 2,2– – bipyridine ligands on each metal terminal, adding electrons to the bipy localized orbital.
Biological activity
The synthesized were evaluated for their antibacterial activity (Table 5) by the cup- plate method. Moderate antibacterial activity was observed for complexes 1, 4 and 6 microorganisms such as Staphylococcus Aureus, Vibrio Cholera and Shigella Flexneri. However some of the complexes show mild antibacterial activity against tested organism.
CONCLUSION
We have reported the synthesis of a novel triazolate bridge ligand and of its binuclear compounds with the Ru-(bipy)2 metal units and studies the spectroscopic and electrochemical properties of such complexes However. The results discussed above have that the triazole nucleus is very interesting bridging ligand. Made clear suggest that the properties of each metal based component are essentially maintained in the (suparmolecular) binuclear arrays. In particular, multi electron redox processes have been evident to occur in the binuclear species, indicating a weak interaction of the metal units across the triazole ring bridged. Such properties make these compounds interesting as potential light-active multi electron transfer catalysts.
In comparison to previous reported Ru (II) complexes most of the newly synthesized Ru (II) complexes show significant at antibacterial activity. 48,49
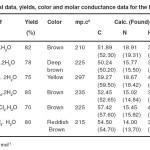 |
Table 1. Analytical data, yields, color and molar conductance data for the Ru (II) complexes Click here to View table |
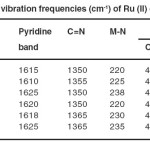 |
Table 2. IR vibration frequencies (cm-1) of Ru (II) complexes. Click here to View table |
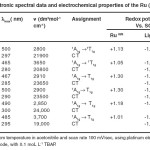 |
Table 3. Electronic spectral data and electrochemical properties of the Ru (II) complexes Click here to View table |
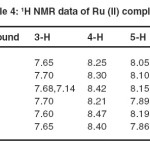 |
Table 4. 1H NMR data of Ru (II) complexes Click here to View table |
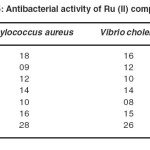 |
Table 5 . Antibacterial activity of Ru (II) complexes Click here to View table |
REFERENCES
- P. J.Van koningsbruggen, J.G. Haasnoot, H. Kooijman, J. Reedijk, A.L.Spek, Inorg. Chem. 1997, 36, 2487- 2489.
- N. Moliner, M.C. Munoz, P.J. Van Koningsbruggen, J.A. Real, Inorg. Chem. Acta 1998 , 274 , 1-6.
- P.J.Van Koningsbruggen, Y. Garcia, E. Codjovi,R. Lapouyade, L. Fournes, O. Kahn, L. Rabarde, J. Mater. Chem. 1997, 7, 2069-2075.
- Z.X. Wang, P. Baiz, J.X. Yang, K.I. Okamoto, X.Z.You, Acta Crystallogr., Sect. C, 1998, 54, 438-442.
- I.P. Passanit, W.R. Browne, F.C. Lynch, D. Hughes, M. Nieuwenhuzen, P. James M.Maestri, J.G. Vos, J. Chem. Soc. Dalton Trans. 2002, 1740-1746.
- A..Juris, V Balzani, F. Barigeletti, S. Campagna, P.Belser, A.V.Zelewsky, . Coord. Chem. Rev. 1988, 84 ,85- 277.
- K. Kalyanasundaram, Coord. Chem. Rev. 1982, 46, 159-244.
- V.Balzani, F. Scandola, Suparmolecular Photochemistry, Ellis Horwood, Chishester, UK, 1991.
- V.Balzani, Suparmolecular photochemistry; (Ed.), Reilel, Dordrecht 1997.
- J.G. Vos, J.M. Kelly Dalton Trans . 2006, 4869-4883.
- C. Sahin, TH Dittrich, C. Varlikli, S. Icli, M.Ch. Lux-Steiner, Sol. Energy Mater. Sol. Cells, 2010, 94, 688- 705.
- P. Tatucuv, M. Rotaru, I. Rau, C. Spinu, A. Kriza, J. Thermal. Anal. Calorim. 2010, 100, 1107-1115.
- G. Satharaj, T. Weyhermuller, B.U. Nair, J. Chem. Crystallogr. 2011 , 41, 353-358.
- K.S.Schanze, K. Sauer, J. Am. Chem. Soc. 1998, 110 ,1180-1186
- K.A. Opperman, S.L. Mecklenburg, T.J. Meyer Inorg. Chem. 1994, 33 ,5295-5301.
- J.Lombard, R. Boulauoche, D.A. Jose, J. Chauvin, M. Collomb, A. Deronzier, Inorg. Chim. Acta 2010 ,363, 234-237.
- 1R.R. Ruminski, T.D.; Aasem, Inorg. Chim. Acta 2010 ,363, 905- 916.
- T.R. Weaver, Unpublished results, the university of North Carolina, Chapel Hill, N.C. 1974.
- M.H. Klingele, S.; Brooker, Eur. J. Org. Chem. 2004, 3422- 3434.
- W.J. Geary, Coord. Chem. Rev. 1971, 7 , 81- 122.
- A.A.G. Al. Hamid, S. Kanan, J. Coord. Chem. 2012, 65(3), 420- 430.
- A.S.AT. DePaula, B.E. Mannan, E. Tfouni, Polyhedron 1999 , 8, 2017-2022.
- A. Marzotto, A. Ciccarese, D.A. Clemente, G.J.; Valle, J. Chem. Soc. Dalton Trans. 1995, 1461-1468.
- I.N.S. Gil, R.H. Nuttrall, D.E. Scarfe, D.W.A. Sharp, J. Inorg. Nucl. Chem. 1961, 18, 79-81.
- R.J.H. Clark, C.S. Williams, Inorg. Chem. 1965 , 4, 350- 357.
- S.P. Ghosh, L.K.J. Mishra, J. Indian Chem. Soc. 1970 ,47, 1154-1166.
- J. Reedijk, D.W. Groeneveld, . Recl. Trav. Chem. Pays-Bas. 1968 ,87, 1079- 1088.
- B. Elias, L.Moucherm, A.K. Mesmaeker, Inorg. Chem. 2007 ,46, 4979- 4988.
- A.B.P. Lever, Inorganic Electronic spectroscopy, 2nd edit., Elsevier, New York 1984.
- G. Muthusamy, K. Natarajan, . Ind. J. Chem. 1995 , 34A, 490- 509.
- K.D. Oyler, F.J. Coughlin, S. Bernhand, J.Am. Chem. Soc. 2007, 129, 210- 216.
- S. Vaduvescu, P.G. Potvin, Eur. J. Inorg. Chem. 2004 , 1763- 1769.
- N. Dharmaraj,K. Natarajan, Ind. J. Chem. 1994 , 33, 785- 789.
- K. Natarajan , R.K. Podder, U. Agarwala, J. Inorg. Nucl. Chem. 1977, 39 , 431-435.
- R. Hag, A.H.J. Dijkhuis, J.G. Haasnoot, J. Reedijk, B.F. Buchanan, J.G. Vos, Inorg. Chem. 1988 , 27, 2185- 2189.
- A.P Halverson, T. AbouELmaaty, L.W. Castle, J. Coord. Chem. 2011 ,64(21), 3693- 3703.
- J.G. Vos , J.G. Haasnoot G. Vos Inorg. Chim. Acta 1983, 71, 155- 162.
- R. Hag , R. Prins , J.G. Haasnoot , R. Reedijk , J.G. Vos , J. Chem. Soc. Dalton Trans. 1987 ,389- 420.
- F.E. Lytle , L.M. Petrosky , L.R. Carlson Anl. Chim. Acta 1981 , 57, 239- 243.
- A.L.Rheingold, P. Saisuwan, N.C. Thomas, Inorg. Chim. Acta 1993, 214,41- 45.
- T.J.Meyer, Pure Appl. Chem. 1986 , 58, 1193- 1206.
- E.M. Kober, J.V. Caspar, B.P. Sullivan, T.J. Meyer, Inorg. Chem. 1988, 27, 4587- 4598.
- F.Cheng, J. Chen, F.N. Tang, L. Chen, J. Coord. Chem. 2012, 65, 2205- 2209.
- L.G. Charbonniere, R.F. Ziessel, C.A.Sams, A. Harriman, Inorg. Chem. 2003, 42, 3466- 3475.
- Y. Wang, W.J. Perez, G.Y. Zheng, D.P. Rillema, Inorg. Chem . 1998, 37, 2051- 2061.
- O. Hass, J.G. Vos, J. Ectroanal. Chem. 1980, 113, 139153.
- B. Durham, J.L. Walsh, C.L. Carter, T.J. Meyer, Inorg. Chem. 1980, 19, 860- 865.
- G.A. Muthukumar, P.Vishwanathamurthi, J. Coord. Chem .2010, 63, 1263- 1272.
- S. Thota, S.S. Kark, K.N. Jayaveera, J. Balzarini, E. DeClercq, J. Coord ,chem. 2010, 63, 4332- 4346.

This work is licensed under a Creative Commons Attribution 4.0 International License.









