Synthetic and Structural Studies of Some Bivalent Transition Metal Complexes with Oxygen and Nitrogen Containing Schiff Base
Ajit Kumar1, U. S. Yadav2 and B. K. Rai*
1J. P. University, Chapra 2University Department of Chemistry, J. P. University, Chapra *Department of Chemistry, L. N. T. College, Muzaffarpur, B.R.A. Bihar University, Muzaffarpur
DOI : http://dx.doi.org/10.13005/ojc/290353
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
The present communication deals with the result of the Schiff base ligand 3-amino 2 ethyl quinazoline 4(3H) Semicarbazone (AEQS) with bivalent transition metal ions, Cu(II), Co(II) and Ni(II). The ligand and its metal complexes are characterized on the basis of molar mass, elemental analyses, IR, electronic spectra, molar conductivity, magnetic moment measurement. The reaction of the ligand with Cu(II), Co(II) and Ni(II) resulted in the formation of the complexes have the general composition [M(AEQS)2]X2 where M= Cu(II), Co(II) and Ni(II). AEQS=3-amino 2 ethyl quinazoline 4(3H) semicarbazone and X = Cl-, Br- or I-. The studies proposes a distorted octahedral geometry for Cu(II) complexes where as octahedral geometry is assigned for Cu(II), Co(II) and Ni(II) complexes.
KEYWORDS:Semicarbozone;AEQS;Cu(II), Co(II) and Ni(II);complexes compound
Download this article as:| Copy the following to cite this article: Kumar A, Yadav U. S, Rai B. K. Synthetic and Structural Studies of Some Bivalent Transition Metal Complexes with Oxygen and Nitrogen Containing Schiff Base. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290353 |
| Copy the following to cite this URL: Kumar A, Yadav U. S, Rai B. K. Synthetic and Structural Studies of Some Bivalent Transition Metal Complexes with Oxygen and Nitrogen Containing Schiff Base. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=368 |
INTRODUCTION
Complexes of semicarbazone ligands exhibit remarkable attention, primarily due to bioinorganic relevance. Semicarbazone complexes exhibit wide medicinal applications such as antimalarial, antiviral, antibacterial, and antitumor1-10. In order to consider above features of semicarbazone Schiff bases ligands and its complexes and in continuation of our recent works11-15 in this field., we herein reported synthesis and characterization of Cu(II), Co(II) and Ni(II) complexes with Schiff base, 3-amino 2- ethyl quniazoline 4(3H) semicarbazone.
EXPERIMENTAL
Analytical and physical data of the ligand and its metal complexes are given in Table-1. The ligand is soluble in common organic solvents, but the complexes are found to be soluble in DMF and DMSO. The molar conductance value in DMF (Table-1) adequately support the 1:2 electrolytic metal of the metal complexes. All the chemicals used were of analytical grade. Metal contents were determined using standard procedures. IR Spectra of the ligand and complexes were recorded on Perkin Elmer model 577 spectrophotometer using KBr disc. Electronic spectra were recorded on Cary-2390 spectrophotometer using DMF as a solvent. Molar conductivity of the complexes were measured by using Systronic conductivity meter. Model 303 in DMF. Magnetic susceptibility was carried out by Gouy method using Hg[Co(NCS)4]
Synthesis of the ligand, AEQS
A hot ethanolic solution of semicarbazide hydrochloride (0.02mol) and an ethanolic solution(20ml) of 3-amino 2-ethyl quinazoline-4(3H) one (0.02mol) were mixed slowly with constant stirring. This mixture was reflused for 4h. On cooling, a colourless compound was precipitated out. It was filtered, washed with cold ethanol and dried in oven m.p. 160+10 C, yield 65%.
Preparation of the complexes
The complexes of Co(II), Ni(II) and Cu(II) have been synthesized by reacting ethanolic solution of the ligand (0.02mol) with respective metal halide (0.01mol). The reaction mixture was refluxed for 4–5 h. On cooling, a coloured compound was precipitated out in each case. The compound was filtered, washed with ethanol and dried in oven. Yield-65-70%.
RESULTS AND DISCUSSION
The important infrared frequencies of the ligand and its complexes along with their tentative assignments are given in Table-2. The IR spectra of the ligand exhibit strong and broad band at 3300 cm-1 assigned17 nN–H vibrations. In the spectra of the complexes this band suffered downward shift proposing coordination through N-atom of Primary amino group. A medium intensity band at 1580 cm-1 in the ligand due to nC=N of azomethine18 is shifted to lower frequencies at 1555 cm-1 upon complexation because of coordination of azomethine nitrogen with metal ions. The enxt IR spectra of the ligand exhibit strong and broad band at 1700 cm-1 assigned19 to nC=O. In the spectra of the complexes this band suffered downward shift clearly indicating coordination through carbohyl oxygen of semicrabazone. The linkage with N atom primary amino group as well as azomethine N and carbohyl/ oxygen is further supported by two far IR spectral bands present at 535-505 cm-1 and 425-395 cm-1 assigned20 to nM–O and nM–N respectively.
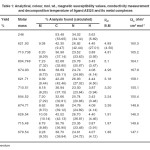 |
Table-1: Analytical, colour, mol. wt., magnetic susceptibility values, conductivity measurement and decomposition temperature of ligand AEQS and its metal complexes Click here to View table |
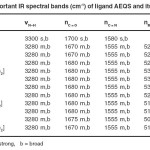 |
Table – 2: Important IR spectral bands (cm-1) of ligand AEQS and its complexes Click here to View table |
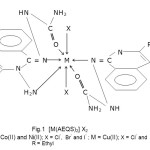 |
Fig.1 [M(AEQS)2] X2M = Co(II) and Ni(II); X = Cl–, Br– and I– ; M = Cu(II); X = Cl– and Br–
R = Ethyl |
Molar Conductivity
The observed molar conductance of complexes of Co(II), Cu(II) and Ni(II) were taken 10-3 DMF solution are in the range 146.3-167.8 ohm-1 cm2 mol-1. The molar conductance values are consistent with the electrolytic21 in nature of 1:2 type.
Electronic spectra and magnetic susceptibility of the complexes
The Co(II) complexes exhibits three spectral bands in the regions 10700-11300cm-1 19330-19500 cm-1 and 23100-23300 cm-1 assigned to 4T2g(F) ¬ 4T1g(F) and 4A2g(F) ¬ 4T1g(F) and 4T1g(P) ¬ 4T1g(F) transitions respectively. The above mentioned spectral bands proposes octahedral22 geometry for Co(II) complexes. The octahedral geometry for Co(II) complexes is further supported23,24 by meff value in the range 4.89-5.13 B.M. The Ni(II) complexes exhibits three absorption bands in the regions, 9700-10200 cm-1, 16100-16400cm-1 and 24300-24700cm-1 assigned to 3T2g(F) ¬ 3A2g(F), 3T1g(F) ¬ 3A2g(F) and 3T1g(P) ¬ 3T2g(P) transitions respectively. The above mentioned spectral bands of Ni(II) complexes proposes octahedral22 geometry. The octahedral geometry of Ni(II) complexes is also supported23,24 by meff value in the range 3.11-3.17 BM. The Cu(II) complexes display two ligands field bands in the regions, 12700-13100 cm-1 and 18200-18430cm-1 assigned 2T2g(F) ¬ 2Eg and charge transferband respectively, which proposes octahedral22 geometry for cu(II) complexes. The magnetic susceptibility23,24 value of Cu(II) complex lies in the range 1.87-1.91 B.M.
Conclusion
The present studies show that the ligand AEQS behaves as neutral tridentate chelating ligand and coordination proposes through amine nitrogen, imine nitrogen and Carbonyl oxygen atom of semicarbozone moiety. Thus on the basis of above mentioned studies it is proposed that the complexes of Co(II) and Ni(II) have octahedral geometry where as distorted octahedral geometry is recorded for Cu(II) complexes [Fig-1].
REFERENCES
- Singh H.L., Sharma M, and Varshney A.K., Synth. React. Inorg. Metal org. Nano-met. Chem, 29, 817(1999).
- Singh R.B. and Ishii H., Crit. Rev. Anal. Chem.; 22, 381(1991).
- Hall I.H., Miller III M.C. and West D.X., Metal based drugs, 4, 89(1997).
- Kasuga N.C. , Shekino K., Ishikawa M., Honda A., Yokoyama M., Nakano S., Shimada N., Koumo C and Nomiya K, J. Inorg. Biochem; 96, 298(2003).
- Saxena A., Tandon J.P., Cancer Lett., 19, 73(1983).
- Graminha A.E., Rodrigus C., Bastista A.A., Teixeira L.R., Fagundes E.S. and Beraldo H., Spectrochim Acta., Part A, 69, 1073(2008).
- Liberta A.E., West D.X. Bio metals, 5, 121(1992).
- Kumar Usha Ashok and Chandra Sulekh, Ttransition Met. Chem., 18, 342(1993).
- Durfee L.D. and Rothwell I.P., Chem., Revi, 89, 1059(1988).
- Tafsumi K., Nakamura A., Huffman P., Stauffert P., and Hoffman R., J. Am. Chem., Soc; 107, 4440(1985).
- Rai B.K. and Anand Rahul, Asian J. Chem., 25, 480(2013).
- Rai B.K., Thakur Amrita and Divya, Asian J. Chem., 25, 583(2013).
- Rai B.K., Vidyarthi S.N., Kumari Punam, Kumari Suman, Kumari Lakshmi and Singh Rajkishore, Asian J. Chem., 25, 941(2013).
- Rai B.K. and Kumar Arun, Asian J. Chem.; 1160(2013).
- Rai B.K., J. Indian Chem., Soc; 90, 105(2013).
- Vogel A.I., A Textbook of Quantitative Chemical Analysis, Revised by Mendhan J., Denny R.C., Barnes J.D. and Thomas M., Pearson education(2008).
- Cook D., Can J. Chem., 39, 2009(1961).
- Agarwal R.K., Agarwal H., Chakraborti I, Synth. React. Met. Org. Chem; 25, 679(1995).
- Saxena R. Gupta S., Rastogi M.K., Asian J Chem., 9, 10(1997).
- Kemp, William “Organic Spectrosciopy”, 3rd Wdition, Polgrave, New York, Silverstein R.M. and
- Diebbar-Sid S., Benali-Baitich O. and Deloume J.P., J. mol. Struct., 569, 121(2001).
- Liver A.B.P., Inorganic electricity spectroscopy, Elsevier, Amsterdam(1968).
- Figgis B.N., Introduction to Ligand Field, Wiely Eastern Ltd., New Delhi, 279(1976).
- Carlin R.L. and Dryneveledt A.J. Van,, Magnetic Properties of Transitions metal compounds, Springer, Verlag, New York (1997).

This work is licensed under a Creative Commons Attribution 4.0 International License.









