Synthesis of some of pharamaceutically important pyrimido annulated analogues of carbazolo and azacarbazolo condensed aza-acridines
Yogita Yadav1, Rajendra singh2, Gurleen Kaur3
1Dept. of chemistry, Banasthali University, Banasthali, Rajasthan,India 2Dept. of Chemistry, I. G.B.N. PG College, Jhunjhunu, Rajasthan India
DOI : http://dx.doi.org/10.13005/ojc/290337
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
This paper describes the synthesis of pyrimido annulated analogues of carbazolo and azacarbazolo condensed aza-acridines respectively by the cyclocondensation of corresponding enol ether,chalcone, oxoketene dithioacetal and dimethyl aminomethylene ketone derivatives with urea, thiourea, acetamidine and guanidine respectively. The structures of all the compounds have been established on the basis of their elemental analysis and (IR, 1HNMR and MS) spectral data.
KEYWORDS:Carbazolo;azacarbazolo;aza-acridines;pyrimido;enol ether;chalcone;oxoketene dithioacetal and dimethyl aminomethylene ketone;IR;1HNMR and MS
Download this article as:| Copy the following to cite this article: Yadav Y, Singh R, Kaur G. Synthesis of some of pharamaceutically important pyrimido annulated analogues of carbazolo and azacarbazolo condensed aza-acridines. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290337 |
| Copy the following to cite this URL: Yadav Y, Singh R, Kaur G. Synthesis of some of pharamaceutically important pyrimido annulated analogues of carbazolo and azacarbazolo condensed aza-acridines. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=335 |
Introduction
The present research work includes to incorporate six membered rings viz; hydroxyl pyrimidine, mercapto pyrimidine, amino pyrimidine, methyl pyrimidine on the carbazole ,azacarbazole and condensed aza-acridine nucleus . The strategy adopted to incorporate these bioactive pharmacophores to this nucleus involves the corresponding enol ethers, chalcones, oxoketenedithioacetals and dimethylaminomethylene ketones respectively. Pyrimidine forms heterocyclic core of DNA and RNA, and has been associated with diverse biological activities.1
Pyrimidines also play a vital role in many biological processes since this ring system is present in several vitamins, coenzymes, nucleic acids etc. therefore Pyrimidines and their derivatives are considered to be important for drugs and agricultural chemicals2,3. This ring system is incorporated into drugs is widely used as anti –viral and for the treatment of AIDS, Cancer. 4-8
The synthesis of this series of heterocyclics was undertaken on this assumption that incorporation of one or more than one bioactive heterocyclic moiety into the pyrimidine framework may result heterocycles with enhanced biological activity.
 |
Fig. 1: Click here to View figure |
Pyrimidine is a promising structural moiety (fig-4.1)for drug designing. Pyrimidine derivatives form a component in a number of useful drugs and are associated with many biological, pharmaceutical and therapeutically activities 15. Condensed pyrimidine derivatives have been reported as anti-microbial16,anti-inflammatory17, anti-HIV18, anti-tubercular19, anti-tumor 20, analgesic21, anti-malarial22, diuretic 23, cardiovascular 24 agents. Pyrimidine compounds are also used as hypnotic drugs for the nervous system 25, calcium-sensing receptor antagonists26 and also for antagonists of the human A2A adenosine receptor27. Drugs such as 5-thiouracil (, gemcitabine , 2-thiouracil , brodimoprin , 5-hydroxymethyl-2-methoxypyrimidine-4-amine , flucystosine , luminal , ritaserin and azidothiamidine(AZT) comprise pharmacological properties such as antineoplastic28, antitumour29, hyperthyroidism30, antibacterial31, antibiotic32, antifungal33, hypnotic34 and anxiolytic35 respectively
Experimental
Melting points were determined on an open capillary and are uncorrected. The IR spectra were recorded on Schimadzu
FTIR-8400S. 1H NMR spectra were recorded in DMSO-d6 and CDCl3 on Brukar 500 spectrometer using TMS as reference, expressed in δ ppm. and Mass spectra were taken on a Bosch Tech – X 600 mass spectrometer at 70 eV.
Preparation of 10-benzyl-5-amino-3-mercapto-3,5,6,9,10,11,12,15-octahydro-2H-[1,6] naphthyridino[3,2-h]quinazolino[4,5a]carbazole-8-carboxylic acid (4.077a)
A mixture of 4.075(1.92g,.004mole),malanonitrile(0.264g,0.004mole) and ammonium acetate(3.08g,0.04mole) in ethanol (10ml) was refluxed on a water bath for 16-18h, cooled, acidified with AcOH and precipitate which settled was collected. It was washed with 30% of aqueous ethanol and solid mass (1.7g) and thiourea (0.25g) was heated on an oil bath at 120oC for 4 hr with constant stirring. The temperature was raised to 1800C and finally the mixture was heated at 220oC for 2 h. On cooling the product solidified, which was recrystallized from DMF-EtOH mixture (1 :2) to give 4.077a 1.49 g Yield 67% m.pt. 295-960C. Similarly 4.077b, 4.077c, 4.077d were prepared from the reaction 4.075b with thiourea and by the reaction 4.075a,4.075b with urea respectively.
Preparation of 10-benzyl-2-mercapto-4-phenyl-3,5,6,9,10,11,12,15-octahydro-2H-[1,6]naphthyridino[3,2-h]pyrimido[4,5a]carbazole-8-carboxylic acid (4.080a)
A mixture of 4.078(0.513g,.001mol),thiourea(0.01g,0.0051mol) and 0.1g NaOH in 25ml of 80% dil.ethanol was refluxed for 1.5h, then
concentrated and cooled, the precipitated 4.080a was filtered off and recrystallized from ethanol. 0.379g. Yield 71% m.pt.245-460C
Preparation of 10-benzyl-4-ethoxy-2-mercapto-3,5,6,9,10,11,12,15-octahydro-2H-[1,6] naphthyridino[3,2-h]pyrimido[4,5a]carbazole-8-carboxylic acid(4.083a)
To a mixture a thiourea (0.152g,0.002mol), sodium ethoxide(1.36g,0.02mol) and ethanol(25-30ml) was added 4.081a(1.058g,.002mol) and the reaction mixture was refluxed for 14 h. The solvent was removed by distillation and residue was treated with glacial acetic acid (7-10ml just enough to dissolved sodium salt of the pyrimidine) and refluxed for 15 minutes. The reaction mixture was acidified with AcOH and precipitate was collected and purified by crystallization with ethanol to give 4.083a 0.789g Yield 76% m.pt. 274-750C.Similarly 4.083b, 4.083c, 4.084d were prepared from reaction of 4.081b on its reaction with thiourea and 4.081a, 4.081b on its reaction with urea respectively.
Preparation of 10-benzyl-2-methyl-6,9,10,11,12,15-hexahydro-5H-[1,6] naphthyridino[3,2h]pyrimido[4,5a]carbazole-8 carboxylic acid(4.086a)
To a solution of 4.084a (0.480g,1mmol) in ethanol (1000 ml) were added acetamidine hydrochloride(0.158g,1.67mmol) and Et3N(2.35ml,1.69mmol) and the solution was heated under reflux for 42 h and concentrated.The residue was extracted with AcOH and was dried over anhydrous MgSO4.The residue was purified by column chromatography eluting with hexane:AcOEt(1:2) to give a brown powder.The solid 4.086a was recrystallized with ethanol to give 0.361g Yield 76% m.pt 224-250C.Similarly 4.086b,4.086c,4.086d were prepared from the reaction of 4.084b with acetamidine and 4.084a,4.084b with guanidine respectively.
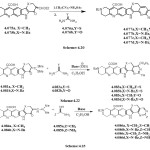 |
Scheme 1 – 3 Click here to View figure |
10-benzyl-5-amino-3-mercapto-3,5,6,9,10,11,12,15-octahydro-2H-[1,6] naphthyridino[3,2-h]quinazolino[4,5a]carbazole-8-carboxylic acid (4.077a).
The compound 4.077a was obtained by applying the general procedure mentioned above. Yield: 67%; m.p. 294–2950 C; IR (KBr) cm−1: 3350.3200,3130,2530,1710,1580,1550,1530, 1020; 1H NMR (300 MHz, CDCl3+DMSO-d6) δ ppm:12.0(1H,s, SH), 11.9 (1H, s, OH), 11.2 (1H,s, NH), 7.35 (asymmetric)[5H,m,ArH]), 6.0(2H,s,NH2], ], 3.7 [2H,s,CH2], 3.62[2H,s,,CH2] 3.1[2H,t,J=6.4 CH2], 2.45[2H,t,J=6.4CH2],2.82[2H,t,J=6.4 CH2],2.76[2H,t,J=6.4CH2] 13C NMR (δ ppm): C (141.2, 124.8, 122.2, 122.0 for indazole ring), CH (128.9, 121.1, 112.0 for indazole ring), C (133.6, 124.1 for indole ring), C (183.0 for carbonyl carbon), CH2 (37.2, 26.4, 24.3 for aliphatic carbons); MS: m/z 559.64 [M+]; Anal. calcd./found for C31H25N7O2S : N, 18.52/18.46.
10-benzyl-2-mercapto-4-phenyl-3,5,6,9,10,11,12,15-octahydro-2H-[
1,6]naphthyridino[3,2-h]pyrimido [4,5a]carbazole-8-carboxylicacid (4.080a).
The compound 4.080a was obtained by applying the general procedure mentioned above. Yield: 65%; m.p. 245–2460C; IR (KBr)cm−1:3300.3400,3010,2600,1600 ,1710,1570,1360; 1H NMR (300 MHz, CDCl3+DMSO-d6) δ ppm:12.9(1H,s, SH), 11.1 (1H, s, OH), 11.9 (1H,s, NH), 8.9[1H,s,ArH],8.27[1H,s,ArH],7.9(asymmetric)[5H,m,ArH],7.25[5H,m,ArH], ], 3.7 [2H,s,CH2], 3.62[2H,s,CH2]3.1[2H,t,J=6.5CH2],2.45[2H,tJ=6.5,CH2],2.82[2H,t,J=6.5CH2],2.76[2H,TJ=6.5,CH2] 13C NMR (δ ppm): C (142.4, 126.3, 124.8, 122.1 indazolering), CH (128.1, 123.1, 112.8 indazole ring), C (186.5,135.3, 113.9 indole ring), CH2 (53.6, 37.8, 33.9 foraliphatic carbon), C (137.4 for 1-benzene), CH (129.1,128.8, 127.9 for benzene ring)MS: m/z 569.19 [M+]; Anal. calcd./found for C34H27N5O2S: 17.71/17.62.
10-benzyl-4-ethoxy-2-mercapto-3 ,5 ,6,9,10,11,12,15-octahydro-2H-[1,6] naphthyridino[3,2-h]pyrimido [4,5a]carbazole-8-carboxylic acid(4.083a).
The compound 4.083 a was obtained by applying the general procedure mentioned above. Yield: 66%; m.p. 274–2740C; IR (KBr) cm−1: 3400.3100,3010,2500,1710,1610,1550,1320; 1H NMR (300 MHz, CDCl3+DMSO-d6)δppm:12.5[H,s,SH],11.9[1H,s,OH], 11.2[1H,s,NH],8.9[1H,s,ArH],8.27[1H,s,ArH],8.1(asymmetric)[5H,m,j=6.7ArH], 4.3[2H,m.j=6.7CH2] , 3.7 [2H,sJ=6.7,CH2], 3.62[2H,s,J=6.7CH2]3.1[2H,t,CH2],2.45 [2H,t,CH2], 2.82 [2H,t,CH2],2.76[2H,t,CH2], 1.33, [3H,t,CH3] 13C NMR (δ ppm):C (143.4, 126.1, 123.7, 121.3 for indazole),CH (128.8, 121.5, 113.1 for indazole), C (132.2,116.1 for indole), CH2 (24.8, 23.3 for aliphatic carbon),128.8, 127.9 for benzene ring)MS: m/z 537.63 [M+]; Anal. calcd./found for C36H32N6O3S: 14.92/14.84…
10-benzyl-2-methyl-6,9,10,11,12,15-hexahydro-5H-[1,6] naphthyridino [3,2-h]pyrimido [4,5a] carbazole-8-carboxylic acid(4.086a)
The compound 4.086 a was obtained by applying the general procedure mentioned above. Yield: 76%; m.p. 224-2250C; IR (KBr)cm−1:3300,3100,3010,1710,1580 ,1550; 1H NMR (300 MHz, CDCl3+DMSO-d6)δppm: 12.1[1H,s,OH],11.8[1H,s,NH],9.5[1H,s,ArH], 9.2[1H,s,CH], 8.27[1H,s,ArH], 7.25[5H,m,ArH], ]3.7 [2H,s,CH2] 3.62[2H,s,CH2]3.1[2H,tJ=6.5CH2],2.45[2H,tJ=6.5,CH2],2.82[2H,t,J=6.5CH2],2.76[2H,t,J=6.5CH2]2.35[3H,s,CH3] 13C NMR (δ ppm):C(141.8, 124.6, 123.7, 121.2 for indazole), CH (128.3,
121.3, 112.1 for indazole), CH2 (56.2, 53.8 foraliphatic carbon), C (137.4 for 1-benzene), CH (128.8,128.5, 128.3, 128.1 127.8 for benzene), C (169.9 for1-carboxyl)MS: m/z 475.54 [M+]; Anal. calcd./found for C29H25N5O2: 14.92/14.84.
Results and Discussion
In the present work, the synthesis of incorporated six membered rings viz; hydroxyl pyrimidine, mercapto pyrimidine, amino pyrimidine, methyl pyrimidine on the carbazole and azacarbazole fused aza-acridine nucleus carried out by the cyclocondensation of corresponding enol ethers, chalcones, oxoketene dithioacetals, and dimethyl aminomethylene ketones with urea, thiourea, acetamide, and guanidine respectively. The enol ethers, chalcones, oxoketene dithioacetals, and dimethyl aminomethylene ketones were synthesized in accordance to the sequence of reaction shown under the heading of the synthesis of starting materials. When enol ethers (4.075a,b), were reacted with malononitrile in the presence of ammonium acetate66 and the resulting o-amino pyridonitrile derivatives were treated with thiourea (4.076a) and urea (4.076b) ,formed the corresponding pyrimidine derivative (4.077a,b,c,d) respectively (Scheme:4.21). Treatment of chalcones (4.078a,b) and oxoketenedithioacetals (4.081a,b) with thiourea and urea, formed the corresponding pyrimidines derivatives (4.080a,b,c,d;4.083a,b,c,d) respectively (Scheme:4.21,Scheme4.22). Reaction of dimethyl aminomethylene derivatives (4.084a,b) with acetamidine (4.085a) and guanidine nitrate(4.085b) hydrochloride, produced the pyrimidine derivatives(4.086a,b,c,d) respectively(Scheme:4.23).
STRUCTURES OF THE COMPOUNDS DISCUSSED
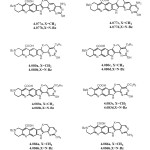 |
STRUCTURES OF THE COMPOUNDS Click here to View figure |
Conclusion
In conclusion, an efficient methodology for the synthesis of pyrimido condensed oxocarbazoles and oxoazacarbazoles and their one-pot conversion to corresponding carbazolo and azacarbazolo fused quinoline carboxylic acids was developed. Heterocyclic scaffolds bearing these structures have been widely studied because of their impressive pharmacological activities. It was, therefore, reasoned that the presence of pyrimidine, carbazole or azacarbazole and quinoline carboxylic acid in tandem with the same molecular framework could produce the novel heterocyclic scaffolds with interesting biological activities
References
- Ghoneim K M and Youssef R, Synthesis and evalution of some 2, 4 and 2,4-disubstituted-6-methylpyrimidine derivatives for antimicrobial activity, J Indian Chem Soc, 1986,53, , 914-917.
- Bhuiyan M. M. H., Rahman K.M.M.; Hossain M.K.; Rahim A,; Hossain,M.I. and Naser M.A.; Synthesis and antimicrobial evaluation of sone new thienopyrimidine derivatives,Acta Pharma.,2006,56,441-450.
- El-Gaby, M.S.A.E. A.; Abdel-Hamide, S.G.; Gnorab M.M. and El-Sayed S.M.; Synthesis and anticancer activity in vitro of some new pyrimidines, Acta Phar, 1999,49,149-158.
- Fauci, A.S. “The Human Immunodeficiency Virus: Infectivity and Mechanisms of Pathogenesis Science, 1988,239,617-622.
- Prince,R., Brew ,B.,Sidtis,J.,Rosenblum,M.,Scheck,A. and P.Cleary.”The Brain in Aids: Central Neerous System HIV-I Infection and Aids Dementia Compex.” Science , 1988,239, ,586-592.
- Ingram, D., Forman, M. and Murray, J. “Phagocytic Activation of Human Neutrophiles by the Detergent Component of Fluosol.”Am.J.Pathol, 1992,140,1081.
- Heaney F., Burke C. and Cunningham D., “Pyrimidine annelated heterocycles-synthesis and cycloaddition of the first pyrimido[1,4]diazepine N-oxides”,J.Chem.Soc.,Perkin Trans ,2001 , I , 622.
- BrownD.J.,Evans R.F. and Cowden W.D., “The Pyrimidine”, Wiley New York,1994.
- Dijick P.V., Clarsen M., Vanderhaehe H. and Somer P.D., “Pyrimidines VII. Chemotherapeutic Properties of hydrazinopyrimidines”, Antibiot Chemoth., 1959,523.
- Lagoja, I.M., Pyrimidines as constituent of natural biologically active compounds, Chemistry and Biodiversity, 2005, 2, 1-50
- Aly, A. A.; Synthesis and pharmacological activity of annelated pyrimidines derivatives; Chinese J. Chemistry; 2004, 23, 211-217.
- Ouf, S. A. and Sherif, S.M., Synthesis and fungiotoxicity of some pyrimidine derivatives, Folica Microbiologica,1993,16,62.
- Irene M. Lagoja.; Pyrimidine as constitutent of Natural Biological Active compounds (Review) Chemistry & Biodiversity 2005, 2,1-50
- Jain MK, Sharnevas SC, Organic chemistry, 2008; 3rd edition; 997-999.

This work is licensed under a Creative Commons Attribution 4.0 International License.









