Synthesis of Diazoketones by the Action of Diazo-n-octane on 4-n Pentyloxy chloride
Niti Saxena1 , Anoop Chandra2, S.Ghazala Imam1 and Geetesh Chandra3
1 Department of chemistry, Bareilly College Bareilly-253005(India) 2Food Safety and Drugs Administration of Uttar Pradesh,Lucknow- 226024 (India) 3 U.P Pollution control Board,Bulandsahar, - 203001 (India)
DOI : http://dx.doi.org/10.13005/ojc/290332
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
The reaction of 4-n Pentyloxy chloride (1 mole ) with higher diazoalkane ( diazo-n-octane,2 mole ) gives 1-Diazo-3-[4ı-n-pentyloxy)-phenyl]-prop -3-one and there characterization are discussed.
KEYWORDS:diazoalkane;4-n Pentyloxy chloride
Download this article as:| Copy the following to cite this article: Saxena N, Chandra A, Imam S. G, Chandra G. Synthesis of Diazoketones by the Action of Diazo-n-octane on 4-n Pentyloxy chloride. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290332 |
| Copy the following to cite this URL: Saxena N, Chandra A, Imam S. G, Chandra G. Synthesis of Diazoketones by the Action of Diazo-n-octane on 4-n Pentyloxy chloride. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=325 |
INTRODUCTION
Diazoalkanes in recent years have been found to be susceptible to various systems and functional groups, such as reactive hydrogen12 ,olefinic and acetylenic bonds13,carbonyl group6 (aldehydic and ketonic ),hydroxyl3 and carboxylic acid chloride groups6 etc, which may be termed as the sites of reactivity towards them.
The action of diazoalkanes on carboxylic acid chlorides or anhydrides produces diazoketones1, 6, 9. Several workers2,4,7,9have studied the action of only lower diazoalkanes on it. A large number of such diazoketones have been synthesized with lower diazoalkanes namely diazomethane and in few cases diazoethane only 9 ,10, 14 by using simple acid chlorides having one site of reactivity. Some work has also been done in laboratory in the past few years on the synthesis of α-diazoketones from carboxylic acid chloride, containing one or more sites of activity towards diazoalkanes. By using different amounts of a diazoalkane, it is possible to attack one or both the sites present in it. By doing so it is possible to compare the reactivities of these sites11.
Therefore attempt was made to synthesise the dizoketones by following the Arndt and Eistert’s method5 by using two molecules of the diazoalkanes per molecule of carboxylic acid chloride. After working out the reactions mixtures, as before the diazoketones (I) was obtained as light yellow thin syrupy liquid. As the diazoketones decomposed on distillation even under vaccum, therefore they could not be obtained in a pure state.
EXPERIMENTAL
Synthesis of 1-Diazo-3-[(4ı-n-pentyloxy) -phenyl]-prop -3-one (I)
It was prepared by using a well cooled ethereal solution of 4-n Pentyloxy chloride (1 mol ) was added in small installment, over a period of half an hour to an ethereal solution of pre-estimated diazo-n-octane (3.2 g ,2 mole) at 0˚ C. The reaction mixture was then kept at 0˚ C, overnight. On removal of ether at low temperature, the diazoketone (I) was obtained as a light yellow thin syrupy liquid (yield 2.4 g) with characteristic odour. It contain nitrogen . As diazoketone, in general decomposed on heating by distillation even under vaccum, hence no attempt was made to purify it.
CHARACTERIZATION
Formation of 2, 4 dinitrophenyl osazone (II) of the diazoketone
The diazoketone with an aqueous alcoholic sulphuric acid solution of 2,4 dinitro phenyl hydrazine gave a 2,4 dinitro phenyl osazone(II) as an orange solid ,which after crystallization from ethanol, melted at 205˚C.
Estimation of elements in the 2,4 dinitrophenylosazone(II)
Found C=48. 35 % H=3.71 % N=16.95 %
C26H26N8 O12 (Requires) C=48.59 % H=4.04 % N=17.44 %
Absorbed frequencies in IR region (solvent KBr) :- 3060 (C-H stretching in aromatic ), 1620 (C=N), 1615 (Benzene ring streching), 1350 (C-NO2 Streching ),724 ( -CH2 rocking in -(CH2)6 CH3 ),2900 cm-1 ( -CH stretching in –CH2)
 |
Fig 1: Click here to View figure |
Action of dry hydrogen chloride
The diazoketone was dissolved in an ether acetone mixture and the solution was kept at 0˚, dry HCl gas then bubbled through this solution for about an hour, till the evolution of nitrogen ceased. After keeping as such overnight, the reaction mixture was poured in water. This organic phase was extracted with ether,washed and dried.On removal of ether, a reddish liquid containing chlorine but no nitrogen was obtained, it was the expected choroketone (III).This when reacted with 2,4 dinitrophenyl hydrazine forms 2,4 dinitro phenyl hydazone (IV) which was crystallised with ethanol, M.P 200 ˚C.
Estimation of elements in the 2,4 dinitrophenylhydrazone (IV)
Found C=50.68% H=3.63% N=10.87% Cl = 7.02%
C20H19O8N4Cl (Requires) C=50.20% H=3.97% N=11.71% Cl =7.32%
Absorbed frequencies in IR region (solvent KBr):-1620 (-C6H5), 1620 (C=N), 720 (-CH2 rock in (-CH2)6 CH3), 675 (C-Cl), 2915 cm-1 (-CH stretching in –CH2)
(C) Action of phenol and benzoic acid
A small amount diazoketone (I ) was treated with molten phenol and benzoic acid separately .The reaction mixture was heated till the evolution of nitrogen ceased. Next day, the excess of phenol and benzoic acid were removed with dilute NaOH and Na2CO3 solution. Then it was extracted with ether, washed and dried. On removal of ether, gummy masses were obtained (V ,VI ), which failed to give any derivative.
Action of silver oxide at 30 ˚ C
A small amount of the diazoketone (I) in dioxin was treated with a freshly prepared silver oxide suspended in water. The reaction mixture was stirred at room temperature for four hours. Next day it was filtered and concentrated until two layers separated .The organic phase was extracted with ether, washed and dried. On removal of ether, a yellow coloured liquid (VII) free nitrogen was obtained, it failed to give any derivative.
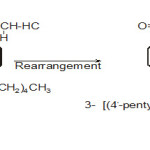 |
Fig 2: Click here to View figure |
Action of Cu powder or UV rays
In the presence of Cu powder or UV rays the diazoketone (I ) dimerises after loss of nitrogen and form (VIII ) namely.
REFERENCES
- Akira; Sato, Takashi ;Ando,Watarce Chem.Lett.1983(7),1084-4 (Eng.).
- A.L.Wilds.,J.Am.Chem.Soc.,84:15 (1962).
- Arndt,Eistert and Partale.,Ber.,60,1364(1927).
- Danheiser,R.L.,Miller,R.F.,Brisbois,R.G.Org.Syn,Coll.,9:197(1988).
- F.Arndt,B.Eistert and W.Partale,Ber,60,1364(1927);61,1949(1928);68,200,212(1935);69,1805(1936).
- L.Wolff,Ann.,23, 394 (1912).
- Muller,360:591 OR, I ,53.
- Robinson and Bradley .J.AmChem.Soc., 50: 1310(1930),52:1558(1928).
- R.P .Kapoor and S.M. Gupta., J.Indian Chem.Soc.,. 38, 776-778(1961).
- Satish Kumar and S.M.Gupta,J.I.C.S.,43,2(1966).
- S.Gupta,P. Garg and A.K.Agarwal .,Oriental Journ Chem ,Vol.25(2),457-458(2009).
- Von Pechmann,Ber.,28,855 (1895); Meyer Monatash,26,1300(1950).
- Von Pechmann,Ber.,31,2950(1898);Arndt and Eistert Ber.,61,1171 (1928),68,196(1935).
- Woliz and Buco,J.Org.Chem.,20,210,(1955).

This work is licensed under a Creative Commons Attribution 4.0 International License.









