Synthesis, Characterization and Semi-empirical study of Di-nuclear Mixed-Ligands Complexes of Ru(II)
F.A.O.Adekunle*, B. Semire and O.A.Odunola
Department of Pure and Applied Chemistry, Ladoke Akintola University of Technology, Ogbomoso, Nigeria.
DOI : http://dx.doi.org/10.13005/ojc/290312
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
The dimeric complexes of Ru(phen)2Cl2.2H2O and Ru(bipy)2Cl2.2H2O prepared from the condensation product obtained by reacting diacetyldihydrazone with 2- acetylpyridine (DDACP) was characterized using microanalyses, infrared and UV-Vis spectra, 1HNMR, mass spectra and the molar conductivity. The elemental analyses data and the mass spectra fragmentation patterns are supportive of the formation of the dimeric mixed-ligand complexes, [Ru(phen)2]2L(ClO4)4. 4H2O and [Ru(bipy)2]2L(ClO4)4. 2H2O. The molar conductances determined, revealed the complexes to be 1:4 electrolytes with values 562 mho cm2mol-1and 575mho cm2mol-1 for the complexes respectively. Semi-empirical method has been used to calculate the binding and stabilization energies of the two complexes which suggested that [Ru(phen)2L]2+is more favoured thermodynamically. Mulliken charges onnitrogen atoms that are involved in coordination show that electrons are transferred from ligands to the Ru(II) ion during coordination.
KEYWORDS:dimeric;complexes;mixed – ligand;fragmentation patterns;semi-empirical method
Download this article as:| Copy the following to cite this article: Adekunle F. A. O, Semire B, Odunola O. A. Synthesis, Characterization and Semi-empirical study of Di-nuclear Mixed-Ligands Complexes of Ru(II). Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290312 |
| Copy the following to cite this URL: Adekunle F. A. O, Semire B, Odunola O. A. Synthesis, Characterization and Semi-empirical study of Di-nuclear Mixed-Ligands Complexes of Ru(II). Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=280 |
INTRODUCTION
The chemistry of polynuclear transitions metal complexes has been of immense current interest, due to the fascinating and versatile properties exhibited by them [1]. Transition metal complexes containing polypyridyl- type ligands exhibit attractive electrochemical and photophysical properties [2 – 8]. The ligand 2, 2’, 6’,2’’-terpyridine has been used widely in the preparation of molecular systems with diverse chemical properties [9]. In the ligand, the position of the nitrogen atom of each pyridine ring allows tridentate and meridian coordination to Ru, Os and Ir [9]. The synthesis of the ligand can be applied to the preparation of derivatives containing an additional functional group which can be used as a bridge to connect two or more different metal centers to produce symmetrical and unsymmetrical metal complexes [9].In addition, much work has been devoted to the bridged bi- and poly-nuclear ruthenium complexes particularly in their mixed valence states [10]. The short-bridging ligands such as pyrazine, tert-butylmalonitrile, dinitrogen, cyanogen or 4,4’-dithiodipyridine provide often a strong enough coupling between ruthenium – amine centers to allow complete electronic delocalization in the mixed – valence (MV) state [10]. Polynuclear complexes of ruthenium(II)are valuable sensitizers for the conversion of light into chemical or electrical energy as they are efficient MLCT chromophores in which the absorption maximum can be tuned to almost any wavelength of the visible spectrum[10]. In order to ensure a potentially good charge separation from the excited state in a donor-chromophore-acceptor triad, it is good to aim at linear, rigid assembly [10].
Herein, we report the synthesis and characterization of the Ru(phen)2Cl2.2H2O and Ru(bipy)2Cl2.2H2O with the condensate of diacetyldihydrazone with 2-acetylpyridine and the semi-empirical study of the complexes investigated.
EXPERIMENTAL
MATERIALS AND METHOD
Reagents
Reagent grade RuCl3.3H2O, 1,10-phenanthroline, LiCl, DMF, diethyl ether, 2,2’-bipyridyl, diacetyl, hydrazine hydrate, glacial acetic acid, 2-acetylpyridine, NaClO4 were purchased from the British Drug House Chemicals Ltd (BDH)and Aldrich Chemicals Co., and were used without further purification.
Preparation of the DDACP
0.456g(1mmole) diacetydihydrazone[11] was transferred into 100mL round-bottom flask, 20mL methanol and 0.90mL2-acetylpyridine added. The mixture stirred with calcium chloride guard-tube fixed to the flask. The bright–yellow solution obtained was stirred for 4hrs, after which the solution was left overnight. The yellow compounds obtained was filtered by suction and dried in the air (0.620g, 48%).
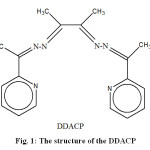 |
Fig. 1: The structure of the DDACP Click here to View figure |
Reaction of Ru(phen)2Cl2.2H2O [12] with DDACP
0.114g(0.2mmole) Cis-Ru(phen)2Cl2.2H2O [12] was transferred to a 100mL two-neck round bottom flask, 1:1 MeOH – H2O mixture(20mL) added and degassed . To this 0.064g (0.2mmole) DDACP was added and the mixture refluxed under N2 gasfor 6hrs. It was then cooled to room temperature and filtered. To the filterate, 0.246g (2mmole) NaClO4solution in 5mL distilled water was added dropwisely with constant stirring. The red precipitate obtained immediately was stirred for 30mins before it was filtered by suction, washed with 15mL diethyl ether and then dried in the vacuo. Yield: 0.07g(20%) [Ru(phen)2]2L(ClO4)4.4H2O Anal. Cal. C66H60N14O20Cl4Ru2(M.M: 1712.718): C, 46.24; H, 3.53; N, 11.45. Found: C, 46.73; H, 3.37; N, 11.40.FTIR: ν/cm-1: 1601 (C=N); 3063(C-H aromatic);625s, 1090vs (ClO4).UV-Vis: cm-122,756; 38,487; 45,274.ESI-MS (CH3CN) m/z: 391.02 (28% {Ru(phen)2L}2+), 310.14 (20% {Ru2(phen)4L}4+), 324.51 (25% {Ru2(phen)4L}4+ .3H2O), 560.99 (20% {Ru2(phen)4(ClO4)2}2+).1H NMR: δ/ppm:2.17(3H, CH3-C-C-CH3), 3.13(3H, CH3-C=N), 7.20 – 8.91 (40H, aromatic).ɅM/mho cm2mol-1562(CH3CN) (1:4 electrolyte).
Reaction of Ru(bipy)2Cl2.2H2O [13] with DDACP
0.064 g (0.2mmole) DDACP was added to 0.104g(0.2mmole) cis-Ru(bipy)2Cl2.2H2O[13]dissolved in degassed 10mL MeOH – H2O mixture(1:1) added and degassed for 30mins. To this 0.064 g (0.2mmole) DDACP was added and the mixture refluxed for 6hrs under N2 gas. It was then cooled to room temperature and filtered. 0. 246g (2mmole) NaClO4solution distilled water (5mL) was added dropwisely with constant stirring. The red precipitate obtained immediately was stirred for 30mins before it was filtered by suction, washed with 15mL diethyl ether and dried in the vacuo. Yield: 0.062g, (20%) [Ru(bipy)2]2L(ClO4)4.4H2O Anal. Cal. C58H56N14O18Cl4Ru2 (M.M: 800.28): C, 44.03; H, 3.57; N, 12.41. Found: C, 43.98; H, 3.67; N, 12.40.FTIR: ν/cm-1: 1600 (C=N); 3070 (C-H aromatic);2972 (C-H aliphatic); 625s, 1090vs (ClO4).UV-Vis: cm-122,437; 23,915sh; 35,452; 39,828sh; 41,787. ESI-MS (CH3CN) m/z: 286.84 (25% {Ru2(bipy)4L}4+),288.82 (28% {Ru(bipy)L}2+), 366.91 (32% {Ru(bipy)2L}2+), 445.82 (18% {Ru(bipy)3L}2+), 674.27{Ru2(bipy)4L(ClO4)2}2+.1H NMR: δ/ppm:2.17(3H, CH3-C-C-CH3), 2.62 (CH3-C=N), 7.49 – 8.84 (40H, aromatic).ɅM/mho cm2mol-1575 (CH3CN) (1:4 electrolyte).
Physical measurements
Microanalyses were performed by a Perkin-Elmer 2400II CHNS analyzer. UV/VIS spectra were recorded on a Perkin-Elmer Lambda 950 spectrophotometer, FTIR spectra(KBr) on a Shimadzu FTIR-8400S spectrometer and ESI mass spectra on a Waters Qtof Micro YA263 Spectrometer. Molar conductances were measured by a Syntronics (India) conductivity meter (model 306) in acetonitrile and 300MHz NMR spectra on a Bruker DPX300 Spectrometer in deuterated dimethylsulphoxide (DMSO-d6).
Results and Discussion
The complexes were obtained by refluxing the Ru(phen)2Cl2.2H2O and Ru(bipy)2Cl2H2O with DDACP in MeOH-H2O mixture under N2. The red precipitates obtained were found to have low yields probably due to the formation of dinuclear Ru(II) complexes in which the L acts as a bridge between two [Ru(phen)2]2+. The microanalyses data for these complexes gave satisfactory results. The colours of the compounds are consistent with those of similar systems [9,14].
The infrared spectra of the complexes revealed the -C=N stretching frequencies at 1601cm-1 and 1600cm-1 respectively for the 1,10-phenanthroline and 2,2’-bipyridine complexes[9]. The aromatic –C-H of both complexes appeared at 3063cm-1 and 3070cm-1 respectively for [Ru(phen)2]2L(ClO4)4. 4H2O and [Ru(bipy)2]2L(ClO4)4.2H2O. The strong bandsat ca 625cm-1 and 1090cm-1observed in both complexescorrespond to the -ClO4stretching frequencies [15].
The single spin-allowed d-d transition from the ground term5T2g to 5Eg [16] was at 22,756cm-1and 22,437cm-1 with a shoulder 23,915cm-1 respectively for [Ru(phen)2]2L(ClO4)4. 4H2O and [Ru(bipy)2]2L(ClO4)4.2H2O. The ligand transitions in the complexes are observed between the range 35,452cm-1– 45,274cm-1 in the two complexes.
The fragmentation patterns observed in the complexes is also an indication that DDACP acts as a bridge between two of the starting Ru(II) compounds.
In the [Ru(phen)2]2L(ClO4)4. 4H2O complex, peaks at m/z = 391.02, 310.14, 324.51and 560.99 correspond to {Ru(phen)2L}2+, {Ru2(phen)4L}4+, {Ru2(phen)4L}4+ .3H2O, {Ru2(phen)4(ClO4)2}2+ respectively. The peaks at m/z = 286.84 and m/z = 288.82were assigned to {Ru2(bipy)4L}4+) and{Ru(bipy)L}2+) respectively in [Ru(bipy)2]2L(ClO4)4. 2H2O while the other peaks in the complex at m/z = 366.91, m/z = 445.82 and m/z = 674.27 stand for {Ru(bipy)2L}2+, {Ru(bipy)3L}2+ and {Ru2(bipy)4L(ClO4)2}2+ respectively. The fragmentation patterns observed in these complexes have molecular weights which were consistent with the expected values.
The 1H NMR of the complexes showed the three methyl protons of (CH3-C-C-CH3) at 2.17ppm for both of them and while the three methyl protons of CH3-C=N- at 3.13ppm and 2.62ppm respectively. The aromatic protons were observed in the complexes between 7.20ppm and 8.91ppm. The number of the aromatic protons in each case indicated that the 1,10-phenanthroline and 2,2’-bipyridine rings in the starting Ru(II) compounds are present in the mixed –ligand complexes coupled with the aromatic ring of the introduced ligand, L. The molar conductance of 562mho cm2mol-1 was obtained for the [Ru(phen)2]2L(ClO4)4. 4H2O complex whilethat of [Ru(bipy)2]2L(ClO4)4.2H2O is at 575mho cm2mol-1. These values showed that the complexes are dimeric.
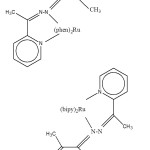 |
Fig. 2: The proposed structure of the complexes Click here to View figure |
Computational methods
Quantum chemical methods (Semi-empirical, PM3) was used for the optimization of the complex geometries. Heat of formation, binding energy for the two mixed-ligand binuclear RuII complexes were also calculated using PM3 as implemented in Spartan 06 computational software package. It has been reported that geometrical parameters from PM3 calculation for transition metal complexes are more accurate than that of ab initio methods [17], however, HF/3-21G* was used to calculate the stabilization energy for the two binuclear Ru(II) complexes.The average Ru1-N (Ru2-N) bond distance are 2.061 (2.068) and 2.061Å (2.063Å) for [Ru2(bipy)4L]4+ and [Ru2(phen)4L]4+ respectively (Table 1).
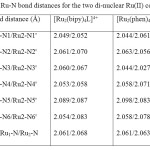 |
Table 1. Ru-N bond distances for the two di-nuclear Ru(II) complexes Click here to View table |
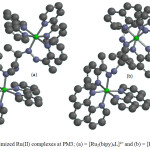 |
Figure 3: Optimized Ru(II) complexes at PM3; (a) = [Ru2(bipy)4L]4+ and (b) = [Ru2(phen)4L]4+: Click here to View figure |
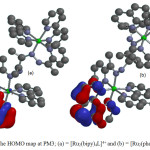 |
Figure 4: The HOMO map at PM3; (a) = [Ru2(bipy)4L]4+ and (b) = [Ru2(phen)4L]4+ Click here to View figure |
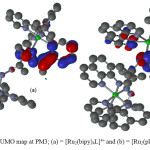 |
Figure 5: The LUMO map at PM3; (a) = [Ru2(bipy)4L]4+ and (b) = [Ru2(phen)4L]4+ Click here to View figure |
Optimized geometries, HOMO and LUMO of [Ru2(bipy)4L]4+ and [Ru2(phen)4L]4+complex ions are shown in Figures 3, 4 and 5 respectively. The highest occupied molecular orbital (HOMO) representing π-electrons of the complexes arelocalized on one unit of 1,10 phenanthroline and 2,2’-bipyridine ligands for [Ru2(phen)4L]4+ and [Ru2(bipy)4L]4+ respectively (Figure 4). The lowest unoccupied molecular orbital (LUMO)is mainly on (1-pyridin-2-yl-ethylidene) -hydrazine subunit of DDACP for the two complexes (Figure 5).The HOMO and LUMOenergies calculated are -17.80 and -9.80 eV for[Ru2(bipy)4L]4+ and -17.15 and -9.59 eV for [Ru2(phen)4L]4+respectively. The EHOMO-ELUMOenergy presenting π – π* main transitions in the complexes are 8.00 and 7.56 eV for [Ru2(bipy)4L]4+ and [Ru2(phen)4L]4+ respectively. The dipole moment (D.M) and polar surface area (PSA) are some important parameters to be considered in solute-solvent interactions which have overall effect on the reactivity, therefore D.M and PSA calculated at Semi-empirical method show that [Ru2(phen)4L]4+ may exhibit more complex-solvent interactions. The Mulliken charges on nitrogen atoms involved in the coordination are all positive and the average charge on these nitrogen atoms are 0.541/0.471e for [Ru2(bipy)4L]4+ and 0.457/0.474e for [Ru2(phen)4L]4+as compared to -0.032/-0.273e and -0.041/-0.223e on N7/N7′ for [Ru2(bipy)4L]4+and [Ru2(phen)4L]4+respectively. These show that electrons are transferred from ligands to the Ru(II) ion during coordination (Table 2).
 |
Table 2: Frontier molecular energies, heat of formation, Mulliken charges, binding and stabilization energies Click here to View table |
To evaluate the effect of DDACP on dinuclear Ru(II) complexes, binding energy (BE) and stabilization energy were calculated from the energy involved in the dissociation processes as shown in equations 1 and 2.
Binding energy (B.E) = [Ru2X4L]4+ – 2E[RuX2]2+ – EL (1)
Stabilization energy (S.E) = E[Ru2X4L]4+ – 2ERu2+ – 4EX – EL (2)
where X = 1,10-phenanthroline or 2,2’-bipyridine and L = DDACP and E = energy of each species.
The calculated binding energy and stabilization energy as presented in Table 2 were carried out only for the ground states of the complex ions. The BE calculated for [Ru2(bipy)4L]4+ are-270.85 and -279.01 kJ/mol for PM3 and HF/3-21G(d) calculations respectively. These are calculated to be -330.16 and -342.94 kJ/mol for [Ru2(phen)4L]4+ at PM3 and HF/3-21G(d) levels. The stabilization energies calculated at HF/3-21G(d) level are -726.82 and -785.01 kJ/mol for [Ru2(bipy)4L]4+ and[Ru2(phen)4L]4+ respectively. Comparison of the binding energies and stabilization energies suggest that [Ru2(phen)4L]4+is more favoured thermodynamically. The higher binding energy and lower stabilization energy in the [Ru2(phen)4L]4+ may be attributed to the availability of more π-electrons on phenanthroline moiety of the compound.
Conclusion
The formation of the bridged mixed – ligand dimeric complexes was supported by the microanalyses, fragmentation pattern in the mass spectra, and the conductivity measurements. The 1HNMR and infrared spectra in conjunction with other analytical data all lends credence to the formation of the compounds.
Furthermore, the quantum chemical methods (Semi-empirical, PM3) used for the optimization of the complex geometries have the HOMO map localized on 1,10- phenanthroline and 2,2’-bipyridine and LUMO is mainly on (1-pyridin-2-yl-ethylidene)-hydrazine subunit of DDACP for the two complexes. The binding and stabilization energiescalculated for the two mixed-ligand dinuclear RuIIcomplexes revealed that [Ru2(phen)4L]4+ion is more stable thermodynamically.
ACKNOWLEGEMENT
F.A.O.Adekunle is grateful to Professor Dipankar Datta of Indian Association for the Cultivation of Science (IACS) Calcutta India for a laboratory space and Third World Academy of Science(TWAS), Trieste, Italy and Indian Association for the Cultivation of Science for a Post-Doctoral Fellowship.
REFERENCES
- DasA.K., ReudaA., FalvelloL.R., Peng S.and BhttacharyaS., Inorg. Chem. 38, 4365 – 4368(1999).
- LeeD. N., ParkH.S., KimE.H., JunY. M., Lee V. LeeW. and KimB. H., Bull. Korean Chem. Soc. 27(1) 99 – 105(2006).
- BennistonA.C., MackieP. R., FarrugaL. J., SmithG., TeatS.J. andMcLean A.J. D., New Journal of Chem. 25, 458 – 464(2001).
- JurisA. and ProdiL., New Journal of Chemistry. 25, 1132 – 1135(2001).
- SuzukiT., KuchiyamaT., KishiS., KaizakiS., TakagiH.D. and KatoM., Inorg. Chem. 42, 785 – 795(2003).
- HutchisonK., MorrisJ. C., NileT.A. and WalshJ. L., Inorg. Chem. 38, 2516 – 2523(1999).
- D’AlocoA., WilliamsR.M., ChriquiY., IyerV.M., BelserP., VergeerF., RuizV., Unwin P. R. and ColaL.D., The Open Inorganic Chemistry Journal. I, 26 – 36(2007).
- WardM. D. Annu.Rep.Prog. Chem., Sect.A, 101, 649 – 672 (2005).
- LopezR., VillagraD., FerraudiG., MoyaS.A. and GuerreroJ., Inorg. Chim. Acta 357 3525 – 3531(2004).
- BonhoteP., LecasA. and AmouyalE., Chem. Commun. 885 – 886(1998).
- FisherH. M. andStouferR. C. Complexes of cobalt(II). Inorg. Chem. 5 (7), 1172 – 1177(1966).
- SullivanB.P., SalmonD.J., MeyerT.J., Inorg. Chem. 17 (12) 3334 -3341 (1978).
- BonnesonP., WalshJ.L., PenningtonW.T., CordesA.W.,Durham B., Inorg. Chem. 22 (12) 1761- 1765(1983).
- DrewM.G.B., NagS. and DattaD. Inorg. Chim. Acta 361, 417 – 421(2008).
- SheeN.K., AdekunleF.A.O., DasD., DrewM.G.B. and DattaD., Inorg. Chim. Acta 375, 101 – 105(2011).
- Lever A.B.P. Inorganic Electronic Spectroscopy; Elsiever, London, edn. 473(1986).
- Hehre W.J. A guide to Molecular Mechanics and Quantum Chemical Calculations. Wavefunction. Inc. USA(2003).

This work is licensed under a Creative Commons Attribution 4.0 International License.









