Synthesis and Characterization of Metal-B-diketonate Coordination Complexes and Polymers
Mohammed A. Al-Anber1,2, Hannen Daoud2
1Department of Environmental Health, Faculty of Public Health and Health Informatics, Hail University, Hail, Saudi Arabia. 2Department of Chemical Science, Mu´tah University, 61710 Al-Karak, P.O. Box 7, Jordan.
DOI : http://dx.doi.org/10.13005/ojc/290307
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
A mononuclear [M(tba)2(H2O)2] (3: M = Mn, 4: M = Ni, 5: M = Zn; tba = deprotonated of 1) complexes have been prepared by the reaction of 3-benzoyl-1.1.1-trifluoro-acetone (H-tba: 1) with M(OAc)2.nH2O (M = Mn, Ni, Zn; OAc = O2CMe) in a 2:1 molar ratio. Complexes 3 - 5 can be extended to form a coordination polymers of general formula [M(tba)2(4,4'-bipy)]n (6: M = Mn, 7: M = Ni, 8: M = Zn; tba = 3-benzoyl-1.1.1-trifluoro-acetone; 4,4'-bipy = 4,4’-bipyridine) by bridging the central metal atom with 4,4’-bipyridine (4,4'-bipy). The reaction progress was controlled via FTIR, UV-Vis spectroscopy and elemental analysis.
KEYWORDS:Manganese;Nickel;Zinc,B-Diketone;3-benzoyl-1.1.1-trifluoro-acetone;FT-IR, 4,4’-bipyridine
Download this article as:| Copy the following to cite this article: Al-Anber M. A, Daoud H. Synthesis and Characterization of Metal-B-diketonate Coordination Complexes and Polymers. Orient J Chem 2013;29(3) |
| Copy the following to cite this URL: Al-Anber M. A, Daoud H. Synthesis and Characterization of Metal-B-diketonate Coordination Complexes and Polymers. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=270 |
Introduction
In the domain of supramolecular chemistry and crystal engineering, metal-organic frameworks (MOFs) and coordination polymers have attracted increasing interests as advanced materials due to their unique properties and multifunctionality [1-2]. MOFs framework structures are formed by the coordination of rod-like bridging ligands, such as pyrazine (pz), 4,4′-bipyridine (4,4′-bipy) etc., to metal centers or metal complex producing 1D-coordination polymeric chains [3-4]. For example, three isomorphic 1D-coordination polymers of [Co(OAc)2(4,4′-bipy)]n (OAc = CH3COO), [Co(H2O)3(4,4′-bipy)SO4].2H2O and [Co(H2O)3(4,4′-bipy)Cl2].2H2O were synthesized and structurally characterized [5]. The solid-state structures for these polymers show one dimensional Co-bipy-Co chains. The polymeric chains of [Co(H2O)3(4,4′-bipy)SO4].2H2O are self-assembly stacked through hydrogen bonds producing a 2D-supramolecular network. Similarly, the polymeric structure of [Co(OAc)2(4,4′-bipy)]n contains linear double Co-bipy-Co chains bridged by CH3COO– groups. From another hand, the synthesis, structure, and reactivity of [Co(acac)(4,4′-bipy)]n [6], [Co(acac)(pz)]n (acac = acetylacetone, pz = pyrazine) [6] and [Cu(acac)(4,4′-bipy)]n [7] polymeric chains were described.
Recently, we have prepared the supermolecule complexes of [Co(tta)]n, [Co(tta)2(H2O)2]n and [Co(tta)2(HOCH3)2]n (tta = deprotonated of 1-thenoyl-4,4,4-trifluoroacetone) [8], whereby the presence of intermolecular MeO−H…Odiketonate hydrogen bridges resulted in the setup of a 1D chain. Through additional p-p interactions between thiophene rings of individual chains a 2D-network structure is generated. Furthermore, the mononuclear [M(tfa)2(H2O)2] complexes have been be extended to form a coordination polymers of general formula [M(tfa)2(4,4′-bipy)]n (M = Mn, Fe, Zn, Co, Ni; 4,4′-bipy = 4,4-bipridine) by bridging the metal atom with 4,4-bipridine (4,4′-bipy) [9 – 10]. In the present work, a mononuclear [M(tba)2(H2O)2] (3: M = Mn, 4: M = Ni, 5: M = Zn; tba = deprotonated of 1) complexes have been prepared. we have extended these complexes to the coordination polymer of [M(tba)2(4,4′-bipy)]n (6: M = Mn, 7: M = Ni, 8: M = Zn; tba = 3-benzoyl-1.1.1-trifluoro-acetone; 4,4′-bipy = 4,4’-bipyridine) by bridging the central metal atom with 4,4’-bipyridine (4,4′-bipy).
Experimental
General remarks
All chemicals were purchased from commercial providers and were used as received.
Physical measurements
Infrared spectra were recorded using a Perkin-Elmer FTIR 1000 spectrometer. Melting points were determined using analytically pure samples with a Gallenkamp MFB 595 010M melting point apparatus. Microanalyses were performed using a Thermo FLASHEA 1112 Series instrument. Thermogravimetric studies were carried out with the Perkin Elmer System Pyris TGA 6 with a constant heating rate of 8 K min-1 under N2 (20.0 dm3 h-1).
Synthesis of [Mn(tba)2(H2O)2] (3):
Complex 3 was prepared by the reaction of Mn(OAc)2.4H2O (48.8 mg, 0.20 mmol) dissolved in 50 ml hot ethanol with Htba (1) (86.3 mg, 0.40 mmol). After 5 hr of stirring at room temperature, the solution dried under vacuum several days and yellow solid is obtained. The product washed several time with petroleum ether and water and dried in oven for 15 min at 50 ºC. M.p: 138-140 ºC. IR (KBr), cm-1: 3437.37 n(O-H) (broad); 1610.66 n(C=O) (Keto-form) (vs); 1577.87 n(C=O) (enol-from); 1188.23, 1136.14 n(C-F) (s); 773.50, 636.55 n(C-CF3) (w). Elemental analysis. Calc. for MnC20O4F6H12.2H2O: C, 46.4; H, 3.0. Found: C, 45.8; H, 3.3 %. λmax (ε): 237 nm (7.2 X 103 L.mol-1.cm-1), 340 nm (9.0 X 103 L.mol-1.cm-1), 365 nm (6.5 X 103 L.mol-1.cm-1).
Synthesis of [Ni(tba)2(H2O)2] (4):
Complex 4 was prepared by the reaction of Ni(OAc)2.4H2O (49.8 mg, 0.20 mmol) dissolved in 50 ml hot ethanol with Htba (1) (86.3 mg, 0.40 mmol). After 5 hr of stirring at room temperature, the solution dried under vacuum several days and green solid is obtained. The product washed several time with petroleum ether and water and dried in oven for 15 min at 50 ºC. M.p: 165 ºC. IR (KBr), cm-1: 3407 n(O-H) (w); 1613 n(C=O) (Keto-form) (vs); 1578 n(C=O) (enol-from); 1188, 1134 n(C-F) (s); 772, 700 n(C-CF3) (m). Elemental analysis. Calc. for NiC20O4F6H12.2H2O: C, 46.1; H, 3.0. Found: C, 46.4; H, 3.2 %. λmax (ε): 237 nm (7.5 X 103 L.mol-1.cm-1), 342 nm (8.2 X 103 L.mol-1.cm-1), 365 nm (6.1 X 103 L.mol-1.cm-1), 565 – 715 nm (very low intensity).
Synthesis of [Zn(tba)2(H2O)2] (5):
Complex 5 was prepared by the reaction of Zn(OAc)2.2H2O (43.9 mg, 0.20 mmol) dissolved in 50 ml hot ethanol with Htba (1) (86.3 mg ,0.40 mmol). After 5 hr of stirring at room temperature, the solution dried under vacuum several days and white solid is obtained. The product washed several time with petroleum ether and water and dried in oven for 15 min at 50 ºC. M.p: 100 ºC. IR (KBr), cm-1: 3437 n(O-H) (broad ); 1613 n(C=O) (Keto-form) (s); 1576 n(C=O) (enol-from); 1462 (s) ;1186, 1136 n(C-F) (s); 775, 700 n(C-CF3) (m). Elemental analysis. Calc. for ZnC20O4F6H12.2H2O: C, 45.5; H, 3.0. Found: C, 39.4; H, 2.8 %. λmax (ε): 246 nm (3.0 X 103 L.mol-1.cm-1), 319 nm (4.4 X 103 L.mol-1.cm-1), 357 nm (3.5 X 103 L.mol-1.cm-1).
Synthesis of [Mn(tba)2(bpy)]n(6):
The Polymer 6 was prepared by the reaction of suitable amounts of monomer [Mn(tba)2.2H2O] (3) with (4.4`-bpy: 2) (70.3 mg, 0.45 mmol) with molar ratio 1:1 in methanol solvent at room temperature for overnight with stirring. The finial yellow participate was washed with methanol and water, and then dried for 15 min in oven at 50 ºC. M.p: 324-326 ºC. IR (KBr), cm-1: 3441 (w); 1611 (vs); 1578; 1535 (m); 1194, 1136 (vs), 774, 718 (w). Elemental analysis. Calc. for MnC30O4N2H20F6: C, 56.2; H, 3.1; N, 4.4. Found: C, 56.3; H, 3.2; N, 4.3 %. λmax (ε): 280 nm (7.4 X 103 L.mol-1.cm-1), 337 nm (9.6 X 103 L.mol-1.cm-1), 480 nm (75 L.mol-1.cm-1), 648 – 739 nm (very low intensity)..
Synthesis of [Ni(tba)2(bpy)]n(7):
The Polymer 7 was prepared by the reaction of suitable amounts of monomer [Ni(tba)2.2H2O] (4) with (4.4`-bpy; 2) (70.3 mg, 0.45 mmol) with molar ratio 1:1 in methanol solvent at room temperature for overnight with stirring. The finial blue participate was washed with methanol and water, and then dried for 15 min in oven at 50 ºC. M.p: >365 ºC. IR (KBr), cm-1: 3445 (w); 1613 (vs) 1578; 1537 (m); 1192, 1138 (vs); 760, 719 (w). Elemental analysis. Calc. for NiC30O4N2H20F6: C, 55.8; H, 3.3; N, 4.3. Found: C, 55.7; H, 3.3; N, 4.3 %.
Synthesis of [Zn(tba)2(bpy)]n(8):
The Polymer 8 was prepared by the reaction of suitable amounts of monomer [Zn(tba)2.2H2O] (5) with (4.4`-bpy; 6) (70.3 mg, 0.45 mmol) with molar ratio 1:1 in methanol solvent at room temperature for overnight with stirring. The finial white participate was washed with methanol and water, and then dried for 15 min in oven at 50 ºC. M.p: 310-315 ºC. IR (KBr), cm-1: 3447 (w); 1611 (vs); 1578; 1539; 1196, 1136 (s); 719 (w); 637 (m). Elemtal analysis. Calc. for ZnC30O4N2H20F6: C, 55.3; H, 3.1; N, 4.3. Found: C, 55.3; H, 3.1; N, 4.2 %. λmax (ε): 235 nm (2.5 X 103 L.mol-1.cm-1), 278 nm (1.8 X 103 L.mol-1.cm-1), 334 nm (6.3 X 103 L.mol-1.cm-1).
Result and discussion
Synthesis and characterization
The reaction of 3-benzoyl-1.1.1-trifluoro-acetone (H-tba: 1) with M(OAc)2.nH2O (M = Mn, Ni, Zn; OAc = O2CMe) in a 2:1 moLar ratio gave the mononuclear [M(tba)2(H2O)2] (3: M = Mn, 4: M = Ni, 5: M = Zn; tba = deprotonated of 1) complexes in ethanol, which was isolated as an yellow, light green and white solids, respectively upon treatment with aqua (Scheme 1). The produced complexes are soluble with most common organic solvent including tetrahydrofuran, acetonitrile, and ethanol. However, in water and non-polar solvents 3 – 5 are not soluble. These complexes are stable in both solution and solid state under the normal conditions. This complex stability had been seen previously in the reported literature’s 22. The gentle heating of the title complex solids, in an oven up to 180 ºC, change the solubility to be non soluble in various organic solvents. The poor solubility indicates for turning into the di- or polynuclear ones by oligomerization through the bridging oxygen atoms of diketonate unit as known and observed of such systems [11].
Complexes 3 – 5 can be extended to infinite metal-organic coordination polymers of [M(tba)2(4,4′-bipy)]n (6: M = Mn, 7: M = Ni, 8: M = Zn; tba = 3-benzoyl-1.1.1-trifluoro-acetone; 4,4′-bipy = 4,4’-bipyridine: 2) by bridging the metal atom in b-diketonate complex spheres with 4,4′-bipy in warm ethanol in a 1:1 molar ratio. These polymers can be prepared directly by stoichiometric reacting of metal acetate with 1 and 4,4′-bipyridine in hot ethanol for 6 hours of reaction stirring (Scheme 1). The aqua ligands in 3 – 5 are eliminated by a strong σ–donor 4,4-bipyridine forming metal-organic coordination polymer. The solutions and solids of polymers are stable in air. After appropriate work-up, polymers 6 – 8 could be isolated as a yellow, light green and white solid, respectively. These polymers are soluble in most common organic solvents including tetrahydrofuran, acetonitrile, and ethanol. However, in water and non-polar solvents these polymers are not soluble.
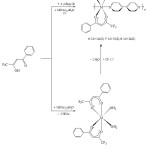 |
Scheme 1. Synthesis of 3 – 8 Click here to View Scheme |
The elemental analyses of 3 – 8 agree with their formula. The chemical nature is characterized by FTIR.
The FTIR spectra of the produced complexes (3 – 5) show prominent peaks. These peaks are found in an area that is typical for metal b-diketone complexes [12]. The FTIR peaks at 1613 and 1576 cm-1 (for 3), 1610 and 1578 cm-1 (for 4) and 1613 and 1578 cm-1 (for 5) are assigned for a keto-enol tautomerism chelating ring of νC=O and νC=C stretching vibrations, respectively [13]. The observed downfield shift, going from free ligand (1: νC=O = 1611 cm-1) to the keto-enol tautomerism chelating ring in 3 – 5 indicates for the complexation, which found inconsistent with the reported one [14]. The presence of these peaks and their shifts (DnC=O) should be regarded as a characteristic stretching vibrations of keto-enol tautomerism chelating ring of tba ligands with M(II) (M = Mn, Ni, Zn) centre as a whole, as in case of benzene [15]. Furthermore, in the FTIR spectrum of 1, the proton peak in an enol chelate-OH appears at 2600-2800 cm-1 [16]. This peak is completely disappeared in the Ft-IR spectrum corresponding of 3 – 5, indicating for the complexation.
The reaction progress of 3 -5 with 4,4’-bipy ligand can be controlled by IR spectroscopy, since the characteristic absorptions of the coordinated aqua ligands in 3 – 5 [17] disappeared during the course of the reaction. This indicates the successful substituting the terminal aqua ligands in 3 – 5 by 4,4′-bipy ligand forming 6 – 8. Generally, IR spectrum shows the prominent absorptions in the range of 1602 – 1412 cm-1 (typical for metal b-diketonate complexes [Experimental Section] [13]).
The UV–Vis spectrum also confirmed the formation of 3 – 5 complexes, wherein the main absorption peaks are summarized in the Experimental section. The main absorption peaks in 1 are 334 nm. This absorption is attributed to π–π* absorption band in the 1–enol form [14 – 15]. Upon coordinating 1 with metal atom (Mn, Ni, Zn) to form 3 – 5, the π–π* absorption band is red-shifted to around 340 nm (for 3), 342 nm (for 4), and 319 nm (for 5). With increasing the concentration of complex we saw one weak broad peak at 565 – 715 nm (for 4), while no similar band appeared in case of 3 and 5. This band corresponds to the d–d transition.
Due to the poor solubility of 4 – 6 polymers the UV-Vis spectroscopy cannot be measured.
Conclusion
The mononuclear complexes 3 – 5 have been successfully prepared and characterized. The produced complexes are extended to infinite metal-organic coordination polymer of formula [M(tba)2(4,4′-bipy)]n (6: M = Mn, 7: M = Ni, 8: M = Zn; tba = 3-benzoyl-1.1.1-trifluoro-acetone; 4,4′-bipy = 4,4’-bipyridine) by bridging the central metal atom in 3 – 5 with 4,4’-bipyridine (4,4′-bipy). The reaction progress have been controlled via FTIR.
References:
- S. A. Bourne, J. J. Lu, A. Mondal, B. Moulton and M. J. Zaworotko, Angew. Chem., Int. Ed. Engl., 40: 2011 (2001).
- B. J. Holiday and C. A. Mirkin, Angew. Chem., Int. Ed. Engl., 40: 2022 (2001).
- A. W. Maverick, F. R. Fronczek, E. F. Maverick, D. R. Billodeaux, Z. T. Cygan and R. A. Isovitsch, Inorg. Chem., 41:6488 (2002).
- J. Lu, C. Yu, T. Niu, T. Paliwala, G. Crisci, F. Somosa and A. J. Jacobson, Inorg. Chem., 37:4637-4640 (1998).
- M.J. Plater, M.R. St. Foreman and A.M.Z. Slawin, Inorg. Chim. Acta, 303:132 (2000).
- B-Q. Ma, S. Gao, T. Yi and G-X. Xu, Polyhedron, 20:1255 (2001).
- Y.Z. Xu and S.Shi, Acta Chim. Sini., 44:336 (1986).
- M. A. Al-Anber, P. Ecorchard, T. Rüffer, H. Lang, in preperation (2013).
- M. Al-Anber, International Journal of Chemical Science and Technology, 3:33 (2013)
- M. Al-Anber, International Journal of Chemical Science and Technology, 3:40 (2013)
- A. I. Matesanz, I. Cuadrado, C. Pastor and P. Souza, Zeitschrift für anorganische und allgemeine Chemie, 631:780 (2005).
- J. Lu, C. Yu, T. Niu, T. Paliwala, G. Crisci, F. Somosa and A. J. Jacobson, Inorg. Chem., 37:4637 (1998).
- B-Q. Ma, S. Gao, T. Yi and G-X. Xu, Polyhedron, 20:1255 (2001).
- C. Chen, D. Xu, Y. Xu and C. Cheng, Acta Crystallogr., Sect. C, 48:1231 (1992).
- M.J. Plater, M.R. St. Foreman and A.M.Z. Slawin, Inorg. Chim. Acta 303:132 (2000).
- M. Al-Anber, P. Ecorchard and H. Lang, Arabian J. Chem. (2011) in press. DOI: 10.1016/j.arabjc.2012.04.048
- M. Al-Anber, P. Ecorchard, T. Rüffer and H. Lang, Main Group Chem., 11:205 (2012).

This work is licensed under a Creative Commons Attribution 4.0 International License.









