Synthesis and Antimicrobial Activity of 1-Thiazolidin-4-ones
Tsegaye Gashaw, Gebremedihin Reda, Hailemichael Ayalew, Neelaiah Babu And Rajkumar Upadhyay*
Department of chemistry, college of natural and computational science, Haramaya University, Ethiopia
DOI : http://dx.doi.org/10.13005/ojc/290314
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
A new series of 2-(3-oxo-3-phenylpropanoyl)-3-(substituted aryl)-1-thiazolidin-4-ones has been synthesized by cyclocondensation of ketoanils (the condensation products of 2, 4-dioxo-4-phenyl butanal with primary amines) with mercaptoacetic acid. The chemical structures of the synthesized compounds were elucidated by elemental analysis, molecular weight determination, and UV, IR, 1H, 13C and DEPT-135 NMR spectral measurements. Bactericidal and fungicidal activates of the new products were studied in virto against Staphylococcus aureus and Eseherichia coli bacteria and Asparigillus niger and Rhizoctia bataticola fungi by using ampicillin and bavistin reference drugs, respectively.
KEYWORDS:Ketoanils; bactericidal; fungicidal; thiazolidinone
Download this article as:| Copy the following to cite this article: Gashaw T, Reda G, Ayalew H, Babu N, Upadhyay R. Synthesis and Antimicrobial Activity of 1-Thiazolidin-4-ones. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290314 |
| Copy the following to cite this URL: Gashaw T, Reda G, Ayalew H, Babu N, Upadhyay R. Synthesis and Antimicrobial Activity of 1-Thiazolidin-4-ones. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=284 |
INTRODUCTION
Imines often called as anils are useful as precursors for the synthesis of variety of organic compounds1-3 including hetrocycles. Thiazolidinones containing sulphur and nitrogen in five member ring, obtained by cyclocondensation of imines with mercaptoacetic acid, are important class of heterocyclic compounds owing to their multifarious applications in diverse disciplines such as in medical science as antibacterial4,5, antifungal6,7, anticonvulsant8, antiubercular9, anti-HIV10, anti cancer11, analgesic12, sadative13, CNS depressant14, hypnotic15 etc agents, in agriculture as herbicidal16, pesticidal17 and insecticidal17, in industries as dyes18-21 and in coordination chemistry as ligands22, 23. The manifold applied features of thiazolidinones in general and broad spectrum of their potent biological activities in particular aroused our interest to synthesize 2-(3-oxo-3-phenylpropanoyl)-3-(substituted aryl)-1-thiazolidin-4-ones by cyclocondensation of ketaoanils (condensation products of 2, 4-dioxo-4-phyenyl butanal with primary amines) with mercaptoacetic acid, characterization by routine physico-chemical methods and evaluate their antimicrobial potential against some Gram-positive and Gram-negative bacteria and fungi, and report in the present communication.
EXPERIMENTAL
Synthesis Scheme
Synthesis of 2-(3-oxo-3-phenylpropanoyl)-3-(substituted aryl)-1-thiazolidin-4-ones involved three steps.
Reagents and conditions
- Selenium dioxide, 96% ethyl alcohol, diethyl ether, refluxed for 5 h.
- Ethanol, reflux 3h.
- Sodium bicarbonate, dry benzene reflux 6h.
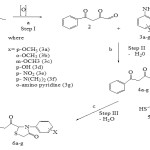 |
Figure 1: Click here to View figure |
Step I: Synthesis of benzoylmethaylglyoxal
Benzoylmethylglyoxal was prepared24 by the partial oxidation of benzoyl acetone with selenium dioxide. Reaction mixture containing benzoyl acetone (1) (0.6 mol) and selenium dioxide (0.6 mol) in 500 ml of 96% ethyl alcohol were refluxed for 5 h. Orange yellow reaction mixture was decanted and concentrated over water bath and after cooling it was dissolved in diethyl ether and selenium was removed by filtration from the product. Solvent of the filtrate was evaporated at room temperature to get dry product and weighed.
Step II: Synthesis of ketonail Schiff’s bases
All benzoylmethyl ketoanils (Schiff’s bases) were prepared24 by mixing equimolar (0.063) saturated solutions of each first step product with substituted aniline, p-nitro, o-methoxy, p-methoxy, m-methoxy, p-hydroxyl and p-dimethyl amino anilines and o-amino pyridine in ethanol and reaction mixtures were refluxed on water bath for 3h and cooled on ice bath and filtered. The solids were washed with diethyl ether.
Step III: Synthesis of 2-(3-oxo-3-phenylpropanoyl)-3-(substituted aryl)-1-thiazolidin-4-ones24
For synthesis of each of 2- (3-oxo-3-phenylpropanoyl)-3-(substituted aryl)-1-thiazolidin -4-ones, to each second step product (0.02mol) thioglycolic acid (0.03 mol) was mixed in dry benzene and the reaction mixtures were refluxed for 6h and hot reaction mixtures were concentrated to half of their volumes over water bath and cooled at room temperature and neutralized with aqueous sodium bicarbonate solution to remove unreacted acid; precipitates of products obtained were filtered and washed with water repeatedly to ensure complete removal of sodium salt(s) and dried in air. All the compounds were purified by crystallization from methanol.
The purity of the synthesized compounds was monitored using thin-layer chromatography. The spots on TLC plates were visualized in iodine vapours and UV light at 254 nm. All the synthesized 2-(3-oxo-3-phenylpropanoyl)-3-(substituted aryl)-1-thiazolidin-4-one products migrated as single spots showed that all products were pure.
Physico-chemical and microbial investigations
Elemental analyses for C, N, H and S contents of the compounds were done on Vario EL-III analyzer. Melting points of compounds determined in open glass capillaries using a digital melting point apparatus are uncorrected. Molecular weights of compounds were determined by Rast`s method using camphor as solvent. Absorption spectra were measured on a Shimadzu UV-2401 Pc UV/Vis spectrometer in 200nm-800nm. IR spectra in 500 cm-1– 4000 cm-1 range were recorded on FT-IR Shimadzu spectrometer in KBr medium. 1H NMR, 13C NMR and DEPT-135 spectra were recorded on Bruker Advance Spectrometer operating at 400 MHz in CDCl3/DMSO solvent.
Antimicrobial (antibacterial and antifungal) activities of the synthesized compounds were tested in vitro using paper disc diffusion method against a few important bacteria like Escherichia coli and Staphylococcus aureus in Mueller Hinton agar (MHA) medium and antifungal activity against common fungi, like Aspargillus niger and Rhizocaticol bataticola in potato dextrose agar (PDA) medium. Known antibiotic ampicillin and fungicidal drug bavistin were used as standard drugs in bactericidal and fungicidal studies respectively. The degree of bacterial and fungicidal activities was determined by measuring diameter of inhibition zones.
RESULTS AND DISCUSSION
Theoretically proposed molecular formulae of the compounds are in conformity with the experimental data of molecular weights and elemental analyses (Table 1).
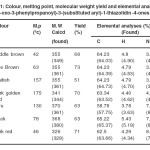 |
Table 1. Colour, melting point, molecular weight yield and elemental analyses of 2- (3-oxo-3-phenylpropanoyl)-3-(substituted aryl)-1-thiazolidin -4-ones (6a-g) Click here to View table |
Both chain and ring C=O chromophoric groups, common in all the synthesized compounds (6a-g) displayed their primary and secondary bands of n→δ* and л→л* electronic transitions in 204-244 nm and 228-314 nm ranges, respectively. O-CH3 auxochromic group of ortho, meta and para methoxy compounds showed л→л* and n→л* electronic transitions in 246-248 nm and 284-348 nm regions, respectively. Both nitro and phenolic groups displayed two bands at 204 nm and 228 nm, and 246 and 354 nm corresponding to n→δ* and л→л*, and л→л* and n→л* electronic transitions, respectively.
The common characteristic groups of 6a-g thiazolidinones exhibit υ C=O (cyclic), υ C-N (ring) and υ C-S-C (ring) vibrations in 1638-1700 cm-1, 1303-1375 cm-1 and 688-715 cm-1 regions, respectively whereas heterocyclic ring CH and methylene group (CH2) coupled with chain CH2 displayed its, δC-H, υ C-H symmetric and υ C-H asymmetric bands in 1425-1487 cm-1, 2836-2889 cm-1and 2912-2953 cm-1 ranges, respectively. The absence of υ CH=N peak of azomethine group of Schiff’s bases observed in 1584 cm-1 – 1589 cm-1 region25,26 and presence of stretching vibrations of characteristic groups of heterocyclic ring obviously confirm the cyclization at the azomethine group of ketoanils with thioglycolic acid. Several other bands corresponding to benzene ring υC=C, υC-H and δC-H vibrations are falling in 1557-1601cm-1& 1438-1530 cm-1, 2999-3120 cm-1 and 688-878 cm-1 and 1005-1325 cm-1 regions, respectively. Diketonic carbonyl aliphatic group vibrations are occurring in 1575-1653 cm-1 range and phenolic and enolic group υO-H, δO-H & υC-O bands are displayed in 3264-3732 cm– 1, 1256-1417 cm-1 & 1005-1120 cm-1 regions respectively; due to presence of methylene group adjacent to carbonyl group enolization is highly probable 27. υC-O-C, υC-H(symm) and υC-H(asymm) vibrations of OCH3 in compound 6a-c are filling in 1048-1176cm-1, 2836cm-1 and 2933-2953cm-1 ranges, respectively and NO2 group in compound 6e also displays bands at 1303 cm-1 (υC-NO2 symm) and 1606 cm-1 (υC-NO2 asymm.
In order to verify infrared inferences regarding the structures of the synthesized compounds, their 1H, 13C and DEPT-135 NMR spectra have been critically examined. 1H NMR spectra of (6a-g) displayed signals for all common proton containing groups, CH2 (aliphatic), CH2 (ring), CH (ring) and benzene ring, in δ 2.2-3.73, δ 2.1-2.23, δ 5.8-6.25 and δ 6.3-8.4 ppm, respectively. Enolic groups signal are displayed in δ 13.0-16.3 ppm invariably in all the compounds most probably due to adjacent position of methylene CH2 group with ketonic group (ring and chain); other substituted groups OH, OCH3 and N (CH3)2 also displayed the characteric signals in δ 6 ppm, δ 3.7-3.9 ppm and δ 3 ppm, respectively.
Perusal of 13C NMR spectra of 6a-g exhibit clear signals of all the common carbon containing groups C=O, CH2 (ring), CH-N (ring) and CH2 (chain), in 156-194 ppm, 20-31 ppm, 31-44 ppm, and 20-55 ppm regions, respectively; two benzene rings twelve carbons displayed their bands in 93-163 ppm range. Pyridine C=N (1C) in 6g depicted its signal at 183 ppm whereas N (CH3)2 (2C) and Ar-OCH3 (1C) exhibited their signals at 41 ppm and 44-55 ppm in their spectra, respectively.
Although in DEPT-135 spectra all the signals for CH2 (chain) and CH2 (ring) are occurring at the same ppm as observed in 13C NMR spectra but in compound 6d and 6f-g instead of downward position as expected some of the signals appeared in upward position; this upward appearance of signals of these groups might be due to enolization of C=O group by shifting of one hydrogen from adjacent methylene group (CH2) in the chain as well as in the ring. The number and chemical shift values of signals of CH (ring) and benzene rings are identical to 13C NMR spectra.
A Perusal of microbial data reveals dose dependent toxicity of the synthesised compounds against all the test microbes. p– Methoxy and o-amino pyridinyl products exhibit moderate significant bactericidal and fungicidal activities against both bacteria and fungi tested. m– Methoxy substituted thiazolidinone is completely inactive against all the microbes. o– Methoxy and p– hydroxy products show moderate antibacterial activity against both bacterial strains and A. niger fungus but non-toxic against R. bataticola fungus. p– Nitro product is active against both bacteria and R. bataticola fungus but inactive against A. niger fungus (Table 2).
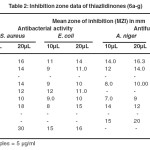 |
Table 2. Inhibition zone data of thiazlidinones (6a-g) Click here to View table |
The structure and activity correlations have been studied among ortho-, meta-, para-methoxy isomers and para substituted N (CH3)2, NO2 and OH thiazolidinones as on para position steric hindrance is minimum. Isomeric methoxy compounds exhibit meta orthopara order in their activities against both of the bacteria and fungi whereas para substituted products fall in order N(CH3)2 NO2 OCH3 and OH N(CH3)2 OCH3 for bactericidal and fungicidal activities, respectively in accordance of electronegativites of substituents.
REERENCES
- Jarrahpour, A. A., Zarei, M. Molbank, M 376: 1-3 (2004).
- Sharma, M. C., Kohli, D.V., Smita Sharma, Sharma, A. D. Der pharmacia Sinica, 1(1): 124 – 125 (2010).
- Gopalakrishnan, S., Nevadith, N. T., Mythili, C.V. Der Chemica Sinnica, 2(3): 124-125 (2010).
- Gebretekle, D., Tadess, A., Upadhyay, R. K., Dekbo, A. Oreintal J. Chem., 28:138-146 (2011).
- Rehaman,V. P. M., Mukhtar, S. H., Ansari, W., Lemiere, G. Eur. J. Med. Chem., 40:173 (2005).
- Singh, G. S., Molotsi, B. J. Farmaco., 60: 723 (2005).
- Verma, S., Srivastava, S. K., Samadhya, P. Intern. J. Pharm. Res. & Dev., 2: 73 (2011).
- Gursoy, A., Terzioglu, N.Turk J. Chem., 29: 247-254 (2005).
- Kucukguzel, S. G., Oruc, E. E., Rollas, S., Sahin, F., Ozbck, A. Eur. J. Med. 37: 197 (2002).
- Rawal, R. K., Prabakar, Y. S., Katti, S. B., Declereg, E. Bioorg. Med. Chem., 13:6771 (2005).
- Dobaria, A.V., Pate, J. R., Parekh, H. H., Indian J. Heterocyclic Chem., 11: 115 (2001)
- Vigorita, M. G., Ottana, R., Monforte, F., Maccari, R., Trovato, A., Monforte M. T., Taviano, M. F. Bioorg. Med. Chem. Lett., 11: 27919 (2001).
- Solankee, A. K., Thakor, I., Patel J., Lad, S. Asian J. Chem., 20: 127 (2004).
- Mishra, P., Namdeo, K. P., Jain, S. K., Jain, S. Asian J. chem., 11: 55 (1991).
- Nair, D. S., Shah, A. C. Indian J. Heterocyclic Chem., 16: 231 (2007).
- Tripathi, D. S., Mishra, A. R., Mishra, R. M., Singh, D., Dwivedi, A. K. Indian J. Heterocyclic Chem., 16: 117 (2006).
- Sinha, R., Garg, S. P., Sah, P. Oriental J. Chem., 20: 131 (2004).
- Upadhyay, R. K., Attri, S., Upadhyay, S., Tiwari, H. K. Asian J. Chem., 23: 3127 (2011).
- Mamo, M., Mengesha, A.T., Upadhyay, R. K., Dekebo, A. J. Pharm. Res., 5: 5543 (2012).
- Upadhyay, R. K., Agarwal, N., Mishra, G. J. Indian Chem. Soc., 72: 849 (1995).
- Krukstis, K. K., Perelman, L. A., Wong, K. Langmuir, 18:10363-10371(1995).
- El-Bindary, A. A., El-Sonbati, A. Z., El-Dissouky, A., Hilali, A. S. Spectrochemica Acta. A: Mol & Biomol. Spectroscopy, 58: 1365 (2002).
- Dave, L. D., Thampy S. K., Thulasidas, S. K. J. Indian Chem. Soc., 58: 1003 (1981).
- Upadhyay, S. Ph. D. Thesis, C, C. S. University, Meerut (India) (2005).
- Bhagat, T. M., Badne, S. G., Kuberkar, S. V., Swamy, D. K. Rasayan J. Chem., 4 (1): 24-28 (2011).
- Saxena, A., Upadhyay, S., Upadhyay, R. K. Sci. Revs. Chem. Commun., 1(1): 63-70 (2011).
- Abdul Khalil, H. P. S., Asniza, M., Issam, A. M. Sains Malaysiana, 40(7): 765–770 (2011).

This work is licensed under a Creative Commons Attribution 4.0 International License.









