Study of Synthesis and Biological Importance of Thiozole (Hetrocyclic Compound)
Parmod Kumar
CCS University, Meerut, UP (INDIA)
DOI : http://dx.doi.org/10.13005/ojc/290357
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
Present study has been carried on synthesis on synthesis and biological importance of hetrocyclic compounds containing thiozole have attracted world wide attention of a large number of chemists ,pharmacologist & biologists on accounts of their significant therophetic and biological properties associated with them. Result of the study shown that they are used as antitubercular, amoebicidal agents , fungicides etc.
KEYWORDS:Hetrocyclic compound;Thiozole;Antimicrobial activity
Download this article as:| Copy the following to cite this article: Kumar P. Study of Synthesis and Biological Importance of Thiozole (Hetrocyclic Compound). Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290357 |
| Copy the following to cite this URL: Kumar P. Study of Synthesis and Biological Importance of Thiozole (Hetrocyclic Compound). Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=376 |
INTRODUCTION
Heterocyclic systems containing thiazole show wide range of activities. The versatility of these nuclei is demonstrated by the fact that some of these compounds exhibit antifungal, antibacterial, antihistaminic, antihyroid and antitubercular activities. The synthetic importance of thiazoles, thiadiazoles, thiadiazines and their condensed heterocyclic systems have been increased much by their recent uses as anthelmintics, antineoplastic, vulcanization accelerators and photographic sensitizers.
Broad – spectrum anthelmintic activity of dl-6-phynyl-2, 3, 5, 6-tetrahydroimidazo [2, 1-b] thiazole hydrochloride (tetramisole hydrochloride) (V)3 against Ascaris lumbricoides and Enterobius vermicularis in man4 and against a variety of adult and immature gastro-intestinal and pulmonary nematodes in laboratory animals, poultry and livestock5 has been reported. Janssen and Coworkers4 found that the condensed thiazole systems possessed more biological activity than its cleavage products. Rudii1 also made the similar observations. In the present investigations, the synthesis of heterocyclic systems, namely, Thiazolo[3, 2-b]-s-triazole, Thiazolo [2, 3-c]-s-triazole, s-Triazolo [3, 4-b][1, 3, 4] have been accomplished..
It was one of the primary aims of the author to study the orientation of cyclization in a situation where there was the possibility of the formation of two isomeric products during cyclization. The orientation of the cyclized product was secured by unequivocal synthesis of thiazolo [2,3-c]-s-triazole system.
SYNTHESIS
General methods for the syntheses of Thiazoles
The synthesis of true thiazoles began with the work of Hofmann48 who prepared 2-chloro and 2-phenylbenzothiazoles. Hantzsch49 was, however, the first to report the synthesis of simple thiazole compounds in a series of papers, beginning from 1887.
After the pioneer work of Hofmann and Hantzsch, the knowledge of thiazole chemistry developed steadily. Bogert and coworkers54 greatly expanded this field. Mills55 in 1922 realized the importance of cyanine dyes containing the thiazole ring as photographic sensitizers. Thus, the commercial importance of benzothiazoles gave impetus to the study of thiazole chemistry.
The general methods for the synthesis of thiazoles are classified on the basis of the fragments of the ring that is contributed by each reactant to build thiazole ring.
Type (i) Synthesis
In this type of synthesis, 1, 5-and 3, 4-bonds of the thiazole ring are formed.
(a) The method first reported by Hantzsch56 and known as Hantzsch thiazole synthesis is the interaction between a-haloketones or a-halogenoaldehydes and thioamide. With proper choice of suitable reactants, thiazoles having alkyl, aryl or heterocyclic substituents attached to any of the three positions (C2, C4 or C5) of the thiazole ring can be synthesised.
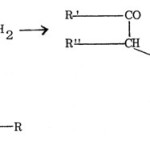 |
Figure Click here to View figure |
Thioamide can be substituted by amide and phosphorus pentasulphide in the synthesis of 2-alkyl-thiazoles57, 58.
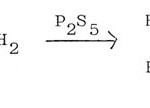 |
Figure Click here to View figure |
Use of salts and esters of dithiocarbamic acid in place of thioamide results in the synthesis of 2-mercaptothiazole 59,60 and 2-alkylmercapto thiazoles61 respectively, whereas the salt and o-esterof monothiocarbamic acid yield 2-hydroxythiazoles62-64 and 2-alkoxythiazoles61, 65 respectively.
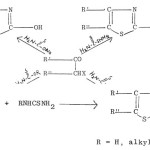 |
Figure Click here to View figure |
King and Miller66 obtained 2-substituted aminothiazoles by using diazoketones in place of a-haloketones in the above reaction.
 |
Figure Click here to View figure |
by treating a ketone (R’ COCH2R”) with thiourea in presence of an oxidant, usually iodine. The halogen can be replaced bythionylchloride, sulphur trioxide, sulphuryl chloride, sulphur monochloride, sulphuric acid, chlorosulphuric acid and nitric acids. a-Haloketone does not seem to be an intermediate since reagents such as SO3, H2SO4 and HNO3 also produce aminothiazoles although in low yield.
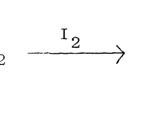 |
Figure Click here to View figure |
Pujari and Jag Mohan made further modification of this method by using N-bromosuccinimide in place of halogen in the synthesis of 2-aminobenzothiazole71.
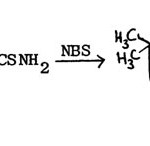 |
Figure Click here to View figure |
Type (ii) synthesis
This type of synthesis involves the formation of 1, 5-and 2,3-bonds of the thiazole ring and includes the preparation of (a) 5-Amino-thiazoles (b) 5-Hydroxythiazoles and (c) Thiazolines.
(a) The reaction of a-aminonitriles with dithioformic acid (salt or ester), carbon disulphide, carbon oxysulphide and isothiocyanates yields 2-alkyl-5- aminothiazoles77, 2-mercapto-5-aminothiazoles78,79, 2-hydroxy-5-aminothiazoles80 and 2-substituted amino-5-aminothiazoles81 respectively.
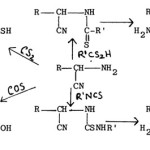 |
Figure Click here to View figure |
The reaction of a-aminoamides with carbon disulphide in presence of a base gives the salt of dithiocarbamic acid, which when treated with acid, undergoes cyclization to give 2-mercapto-5-hydroxy-thiazole (2-mercapto-5-thiazolidone)79,82.
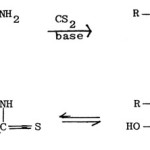 |
Figure Click here to View figure |
(b) 5-Hydroxythiazoles83,84 are obtained by the cyclization of N-thioacyl derivatives of glycine with PCI5 or Ac2O.
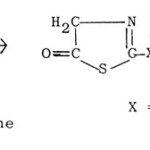 |
Figure Click here to View figure |
EXPERIMENTAL
All melting points are uncorrected. Thin layer chromatography (tlc) was performed on BDH silica-gel (G) plates using acetone-benzene (1 : 3) as solvent system IR and PMR spectra were recorded on the Beckman IR-20 and Perikin-Elmer 90MHz instruments respectively. [n max in cm–1 and chemical shifts in d (ppm) downfield from TMS].
3- a-Naphthyl-5-mercapto-s-triazole (II)
1- a-Naphthoyl-3-thiosemicarbazide (I, 8.2 g, 0.033 mole) in 8% sodium hydroxide solution (100 ml) was refluxed for about 3hr. The reaction mixture was cooled to the room temperature and acidified with dilute acetic acid. The product separated was filtered, washed with water and crystallised from ethanol as colourless shining needles, yield 4.3 g (57%), m.p. 2640 [Found : N, 18.96; S, 14.58. C12Hm9N3S requires N, 18.50; S, 14.19%] ; IR : 755, 780, 1590, 1610, 2580, 3060.
3- a-Naphthyl-5-p-nitrobenzoylmethylmercapto-s-triazole (IIIa, R=p-O2N-C6H4-)
A mixture of II (2.27 g, 0.01 mole) and p-nitrophenacyl bromide (2.44g, 0.01 mole) in anhydrous ethanol (200ml) and DMF (10 ml) was heated under reflux for 5 hr. Cooled to the room temperature and neutralised with aqueous potassium carbonate solution. The yellow solid separated was filtered, washed with water and crystallised from ethanol as light yellow needles, m.p.2120, yield =2.09 (52%) [Found : S, 8.62. C20H14N4O3S requires S, 8.21%]; IR : 765, 790, 840, 1350, 1535, 1570, 1610, 1690, 3150, 3400.
Similarly, the following compounds were prepared IIIb (R=p-CI-C6H4-) : m.p. 1500, yield 63% [ Found : S, 9.12. C20H14N3OSCI requires S, 8.43%] ; IR : 715, 770, 800, 830, 1575, 1620, 1695, 3450.
IIIc (R = p-Br-C6H4-) : m.p. 1850, yield 66% [Found : S, 7.08. C20H14N3OSBr requires S, 7.55%]; IR : 720, 780, 800, 535, 1535, 1680, 3220.
2- a-Naphthyl-5-p-nitrophenylthiazolo [3,2-b]-s-triazole (IVa, R=p-O2N-C6H4-)
A mixture of IIIa (1.0 g), P2O5 (4g) and H3PO4 (3 ml) was heated on an oil-bath at 1500 for about 3hr. The reaction mixture was cooled, poured into water and neutralized with aqueous potassium carbonate solution. The solid thus obtained was filtered, washed with water and crystallized from DMF-ethanol mixture as greenish yellow granules, m.p. 1980, yield 0.43 g (35%) [Found : C, 64.81; H, 3.44; s, 8.80. C20H12N4O2S requires C, 64.52; H, 3.23; S,8.60%] ; IR : 765, 790, 840, 1500, 1540, 1590, 3100, 3125; PMR (TFA): 7.45 (1H, s, C6-H), 7.57 [1H, dd, C2, –H, J2’, 3’ = 7.2 Hz (ortho-coupling), J2’,4’ = 2.7 Hz (meta-coupling)], 7.80-8.02 [4H, m, C3’ –H, C6’–H and C7’–H], 8.17 [1H, dd, C5’-H, J5’,6’ 7.2 Hz (ortho-coupling), J5’,7’ 2.7 Hz (meta-coupling)], 8.43 (4H, q, J=9 Hz, p- nitrophenyl protons), 8.98 [1H, dd, C8’–H, J7’,8’=7.2.Hz (ortho-coupling), J6’, 8’ = 2.7 Hz (meta-coupling)].
Other compounds prepared similarly were : IVb (R = p-C1-C6H4–) : Yield 63%, m.p. 1500 [Found: c, 66.60; H, 3.46; N, 12.02; S, 9.10. C20H12N3SCI requires C, 66.39; H, 3.32; N, 11.62, S, 8.85%]; IR : 760, 780, 800, 825, 1640, 3100.
IVc (R = p-Br-C6H4-) : Yield 52%, m.p. 1660 [Found : C, 58.85. H, 3.10; N, 9.86; S, 8.32. C20H12N3SBr requires C, 59.11; H, 2.96; N, 10.34; S, 7.88%]; IR : 730, 770, 800, 830, 1640, 3120.
3-a-Naphthyl-5-p-nitrophenylthiazolo [2, 3-c]-s-triazole (Via, R=p-O2N–C6H4–)
A mixture of Va (1 g)and POCI3 was heated on an oil-bath (120-1300)for 3hr. The reaction mixture was cooled, poured into water and neutralized with aqueous potassium carbonate solution. The solid thus obtained was filtered, washed with water and crystallized from ethanol mixture as yellow crystals, m.p. 2330, yield 0.43 g (42%) [Found : C, 64.24; H, 3.42; s, 7.89. C20H12N4O2S requires C, 64.52; H, 3.23; S,8.60%] ; IR : 725, 780, 820, 1625; PMR (TFA): 7.76 (1H, s, C6-H), 7.90 [1H, dd, C2, –H), 8.02-8.52 [9H, m, C3’ –H, C4’–H, C6’–H, C7’–H, C5’–H and p-nitrophenyl protons), 8.72 [1H, dd, C8’–H)
Other compounds prepared similarly were : VIb (R = p-C1-C6H4–) : Yield 64%, m.p. 1660 [Found: C, 66.72; H, 3.61; N, 12.00; S, 9.47. C20H12N3SCI requires C, 66.39; H, 3.32; N, 11.72, S, 8.85%]; IR : 735, 775, 810, 1625.
IVc (R = p-Br-C6H4-) : Yield 35%, m.p. 1860 [Found : C, 58.85. H, 3.39; N, 9.87; S, 8.24. C20H12N3SBr requires C, 59.11; H, 2.96; N, 10.34; S, 7.88%]; IR : 725, 770, 840, 830, 1620.
EVALUATION OF USEFUL BIOLOGICAL IMPORTANCE
Relative merits of the compounds as anti-bacterials.
The compounds, (R = p-Br-C6H4–), (Ar = p-Br-C6H4–),and (R = p-Cl-C6H4–) pertaining to the following systems have been evaluated for their anti-bacterial activity. The results of antibacterial screening and the conclusions derived from them regarding the relationship between anti-bacterial activity and the structural changes are summarized.
- Thiazolo [3, 2-b]-s-triazole
- Thiazolo [3, 2-c]-s-triazole
The most widely used method for determining the anti-bacterial activity of drugs consists of cultivating the bacteria in a test tube or nutrient agar plate to which the drug has been added. Factors which influence the results of any test method include (i) species of test organism (ii) composition and pH of the medium (iii) inoculum of organism (iv) diluting fluid (v) concentration and stability of the drug solution and (vi) temperature and duration of incubation.
For studying the anti-bacterial properties, many methods are available but Kirby-Barr disc diffusion and plate dilution methods as reported by Nakahara et al.163 has been used in the present investigations.
The test organism was a two-hour culture of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa incubated and grown in peptonewater medium at370.
ANTI-BACTERIAL TESTING
(Experimental results and conclusions derived therefrom)
Incubation period – 18 hr.
Temperature – 370C
M.I.C. – Minimum inhibitory concentration.
E.coli. – Escherichia coli (NCTC 10418)
S.aureus – Staphylococcus aureus (NCTC 6571)
Ps. aeruginosa – Pseudomonas aeruginosa
(NCTC 10662)
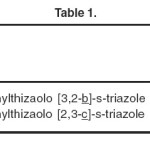 |
Table 1: Click here to View table |
Relative merits of the compounds as antifungals.
METHOD USED
The compounds which were tested for anti-bacterial activity have also been studied for their antifungal action by bio-assay. Kirby-Barr disc diffusion and plate dilution methods as reported by Nakhara et al.163 has been used by the author in the present investigations.
Bio-assay aims at determining the fungistatic or fungicidal efficiency of a compound to figures of performance that can be compared with similar data obtained from other fungistatics of fungicides. It is useful in the evaluation of fungicides since chemistry of the process by which the fungicides function is not yet thoroughly studied. Candida albicans is a most common human pathogen. Therefore, it was used as a fungus strain..
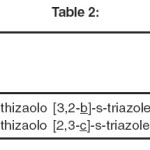 |
Table-II Click here to View table |
CONCLUSION
Comparison of the antibacterial and antifungal activity of the compounds IVc and Vic reveals that the two isomeric systems namely thiazolo (3,2-b]-s-triazole and thiazolo [2, 3-c]-s-triazole posses the same activity. The compounds were found active against S.aureus / C.albicans., when tested as neat samples and may be used for local application in the form of powder of ointment provided further studies indicate absence of toxicity following local application. In the present investigation, the author has synthesised various types of heterocyclic compounds containing a thiazole, has been achieved.
REFERENCES
- R.V. Rudii, Zb. Nauk. Prats Lvivs’k Med. Inst., 24, 47(1963); Chem. Abstr., 62, 16849 (1965).
- E.F. Elslager and P.E.Thompson, Ann. Rev. Pharmacol., 2, 193 (1962); Chem . Abstr., 57, 2795 (1962).
- A.H.M. Raeymaekers, F.T.N.Allewijn, J. Vandenberk, P.J.A. Demoen, T.T.T. Van Offenwert and P.A.J. Janssen, J.Med. Chem., 9, 545 (1966); Chem. Abstr., 65, 3856 (1966).
- D.Thienpont, O.F.J. Vanparijs, A.H.M. Raeymaekers, J.Vandenberk, P.J.A. Demoen, F.T.N. Allewijn, R.P.H. Marsboom, C.J.E. Niemegeers, K.H.L. Schellekens and P.A.J. Janssen, Nature, 209, 1084 (1966); Chem. Abstr., 64, 20473 (1966).
- J.K.Walley, Vet. Record., 78, 406 (1966).
- R.Junghaehnel, G.Renchkhoff and K.Thewalt, Fr. Pat., 1, 585, 632 (1970); Chem. Abstr., 74, 41427r (1971).
- G.Mazzone, F.Bonina, G. Puglisi, R.R. Arrigo, C. Cosentino and G. Blandino, Farmaco, Ed. Sci., 37, 685 (1982); Chem. Abstr., 98, 100754r (1983).
- E.F.Elslager, in “Annual Reports in Medicinal Chemistry”, 1965, p. 142 (Ed. C.K. Cain), Academic Press, N.Y. 1961.
- H.Nesvadha (Sandoz Ltd.) Swiss Pat., 577, 503 (1976); Chem. Abstr., 85, 177400 m (1976).
- E.F. Elslager and D.F. Worth, U.S. Pat., 3, 839, 348 (1974); Chem. Abstr., 82, 43400r (1975).
- Masao Tsuruoka, Igaku to Seibutsugaku (Med. and Biol.) 10, 296 (1947); Chem. Abstr., 47, 2266 (1953).
- R.L. Mayer, M.R. Grimm and F.C. Kull, Proc. Soc., Exptl, Biol. Med., 81, 163 (1952); Chem. Abstr., 47, 1213 (1953).
- G.Sakaki, Ann. Rept. Research Inst. Tuberc., Kanazawa Univ., 11 No. 1, 39 (1953); Chem. Abstr., 48, 7790 (1954).
- M.M.Kochhar and B.B. Willams, J. Med. Chem., 15, 322 (1972).
- S.Kano and T. Noguchi, Japan Pat., 71, 37, 836 (1971); Chem. Abstr., 76, 25295g (1972).
- K. Yokoo, S. Ishiguro and T.Shishido, Japan Kokai., 76, 102, 639 (1976); Chem. Abstr., 86, 49184 (1977).
- H.G.Rogers, Ger. Pat., 1,137,756 (1962); Chem. Abstr., 58, 9787 (1963).
- C.Leiberman and A. Lange, Ber. 12, 1588 (1879).
- A.W.Hoffman, Ber., 12, 1126, 2359 (1879); Ber., 13, 8 (1880).
- Green J. Soc. Chem. Ind., 7, 179 (1888); Ber., 22, 968 (1889).
- M.T. Bogert and E.M. Abrahamson, J. Amer. Chem. Soc., 44, 826, (1922); Chem. Abstr., 16, 1587 (1922); M.T.Bogert and M. Meyer, J. Amer. Chem.

This work is licensed under a Creative Commons Attribution 4.0 International License.









