Reduction of Aldehydes and Ketones with LiBH4 Under Microwave Irradiation
Elnaz Latifi Mamaghani and Davood Setamdideh*
Department of Chemistry, Faculty of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, 59135-443, Iran.
DOI : http://dx.doi.org/10.13005/ojc/290313
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
A variety of aldehydes and ketones have been reduced to their corresponding alcohols within 30-240secondswith excellent yields (92-97%) of products by LiBH4 under microwave irradiation in H2O as green solvent.
KEYWORDS:Aldehyde;Ketone;LiBH4;Microwave;Alcohol;H2O
Download this article as:| Copy the following to cite this article: Mamaghani E. L, Setamdideh D. Reduction of Aldehydes and Ketones with LiBH4 Under Microwave Irradiation. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290313 |
| Copy the following to cite this URL: Mamaghani E. L, Setamdideh D. Reduction of Aldehydes and Ketones with LiBH4 Under Microwave Irradiation. Orient J Chem 2013;29(3). Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=282 |
Introduction
Alcohols are important in organic synthesis.We have reported some reducing systems for the preparation of alcohols from the corresponding carbonyl compounds such as NaBH4/C1, NaBH4/M.W2, NaBH4/Al2O33, NaBH4/TiO24, NaBH4/(NH4)2C2O45, NaBH4/Ba(OAc)26,NaBH4/DOWEX(R)50WX47, Zn(BH4)2/H2O8, Zn(BH4)2/Al2O39, Zn(BH4)2/C10, Zn(BH4)2/2NaCl11, Zn(NH4)2/ZrCl412, and reagents such as [Zn(BH4)2.py]13, [Zn(BH4)(nmi)]14, [Zn(BH4)2.nic)]15. In this context, we now wish to report an efficient, facile preparation of alcohols using aldehydes and ketones by LiBH4/Microwave system in H2Oas green solvent.
Results and Discussions
Microwave irradiation as an unconventional energy source has been used to carry out many kinds of chemical reactions. The microwave irradiation drives chemical reactions effectively and quickly16-17. The model reaction has been selected by reduction of benzaldehyde. This reaction was carried out in H2O (5 mL) as green solvent, different amounts of LiBH4 and different power amplitude of microwave ovenfor the selection of appropriate conditions. The optimization reaction conditions showed that using 1 molar equivalents of LiBH4and 30% power amplitude of microwave oven (300 W) was the best for reduction reaction.The reaction was completed in 30 sec and benzyl alcohol was obtained in 95% yield as shown in scheme 1.
 |
Scheme 1 Click here to View table |
The efficiency of this protocol was further examined by using various structurally different aldehydes. In this approach, the correspondingalcohols were obtained in excellent yields (92-97%) within 30 sec.as shown in Table 1(entries 1-8).In the next attempt, the reduction of ketones has been investigated. The reductionof ketones, because of their less reactivity needs the use of 2 molar equivalents of LiBH4. A variety of ketoneswere subjected to LiBH4 inwater(5 mL) under microwave irradiation (300 W).The results showedthat the corresponding secondary alcohols were obtained inexcellent yields (94-97%) within 80-240 sec as shown in Table 1 (entries 9-13).Addition of distilledwater to the reaction mixture and then extracting with CH2Cl2afforded the crude corresponding alcohol.
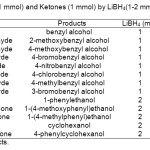 |
TABLE 1:Reduction of Aldehydes (1 mmol) and Ketones (1 mmol) by LiBH4(1-2 mmol) in H2O (5 mL) Under Microwave Irradiation (300 W). Click here to View table |
Experimental
All microwave assisted reactions were carried out in aYusch household microwave oven (1000W). The instrumentwas modified for laboratory applications with an external reflux condenser.IR and 1H NMR spectra were recorded on PerkinElmer FT-IR RXI and 400 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples (melting or boiling points). TLC was applied for the purity determination of substrates, products and reaction monitoring over silica gel 60 F254 aluminum sheet.
Reduction of benzaldehydewith LiBH4/Microwave Irradiation, A typical procedure:
In a round-bottomed flask (10 mL) charged with distilled water (5 mL), LiBH4 (0.022 g, 1mmol) and benzaldehyde (0.106 g, 1 mmol) was added. After fitting the flask to the external condenser at the inside of the oven, the mixture was irradiatedwith a microwave oven (30% power amplitude, 300 W) for30 sec. The progress of the reaction was monitored by TLC(eluent; CCl4/Et2O: 5/2). At the end of the reduction, distilledwater (5 mL) was added to the reaction mixture and it wasthen extracted with CH2Cl2(2×10 mL). The combined extractswere dried over anhydrous sodium sulfate. Evaporation ofthe solvent afforded the pure liquid benzyl alcohol (0.102 g, 95%).
Conclusion
In this context, we have shown that a variety of aldehydes and ketoneshave been reduced to their corresponding alcohols with lithiumborohydride under microwave irradiation. Thereductions were completed within 30-240 sec with excellent yields of the corresponding alcohols. Therefore, this protocol with the easy work-up procedure could be a usefuladdition to the present methodologies.
Acknowledgements
The authors gratefully appreciated the financial support of this, work by the research council of Islamic Azad University branch of Mahabad.
References
- Zeynizadeh, B.; Setamdideh, D. Z. Naturforsch. 61b: 1275 (2006).
- Zeynizadeh, B.; Setamdideh, D.J. Chin. Chem. Soc.52: 1179 (2005).
- Zeynizadeh, B.; Setamdideh, D. Asian. J. Chem.21:3588 (2009).
- Setamdideh, D.; Krimi, Z. Rahimi, F. Orient. J. Chem.27:1621 (2011).
- Setamdideh, D.; Ghahremani, S. S. Afr. J. Chem.65:91 (2012).
- Mohamadi, M.;Setamdideh, D.;Khezri, B.Org.Chem. Inter.doi:10.1155/2013/127585 (2013).
- Setamdideh, D.; Khezri, B.; Alipouramjad, A. J. Chin. Chem. Soc.60: 590 (2013).
- Setamdideh, D.;Khezri, B.;Rahmatollahzadeh, M.;Aliporamjad, A.Asian J. Chem. 24:3591(2012).
- Setamdideh, D.;Khezri, B.;Rahmatollahzadeh, M.J. Serb. Chem. Soc.,79:1 (2013).
- Setamdideh, D.;Rahmatollahzadeh, M.J. Mex. Chem. Soc.,56:169 (2012).
- Setamdideh, D.; Khaledi, L. S. Afr. J. Chem. 66:150 (2013).
- Kamari, R.; Setamdideh, D. Orient. J. Chem.29:497 (2013).
- Zeynizadeh, B.; Setamdideh, D.; Faraji, F. Bull. Korean Chem. Soc.79: 295 (2006).
- Zeynizadeh, B.; Setamdideh, D.Asian. J. Chem.21:3603 (2009).
- Setamdideh, D.; Rafig, M. E-J. Chem.9: 2338 (2012).
- Loupy, A. Microwaves in Organic Synthesis; JohnWiley& Sons: New York, (2002)
- Lidstrom, P.; Tierney, J.;Wathey, B.; Westman, J. Tetrahedron57: 9225 (2001).

This work is licensed under a Creative Commons Attribution 4.0 International License.









