Phytochemical Studies from the Roots of Girardinia heterophylla
NishaTripathi*1, Sunita Kumar1, Rakesh Singh2 C.J.Singh3, Prashant Singh4 and V.K.Varshney5
1Department of Chemistry, M.K.P.P.G. College, Dehradun, Uttarakhand 2Deprtment of Chemistry, D.B.S.P.G. College, Dehradun, Uttarakhand 3Forest Conservation Division, Ministry of Environment and Forest, Delhi 4Department of Chemistry, D.A.V.P.G. College, Dehradun, Uttarakhand 5Chemistry Division, Forest Research Institute (FRI), Dehradun, Uttarakhand
DOI : http://dx.doi.org/10.13005/ojc/290342
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
Girardinia heterophylla (Family: Urticaceae) roots has not been studied so far. The swollen base of roots were collected from and extracted with petroleum ether. The dried petroleum extract was subjected to column chromatography and TLC. Three compounds were isolated from the roots of Girardinia heterophylla. On the basis of spectral analysis they were identified as β-sitosterol, γ-sitosterol and ursolic acid. In this study the presence of γ-sitosterol and Ursolic acid in roots of Girardinia heterophylla has been reported for the first time.
KEYWORDS:Girardinia heterophylla;β-sitosterol;γ-sitosterol;ursolic acid
Download this article as:| Copy the following to cite this article: |
| Copy the following to cite this URL: |
Introduction
Girardinia heterophylla, commonly known as ‘DansKandali’, is abundantly found in the Himalayas. It can be seen growing extensively as an underutilized biomass in the forest areas situated in outskirts of the villages.Swollen base of roots of Girardinia heterophylla is used as soap/shampoo for washing. The leaves of this plant are boiled and cooked for vegetables. The whole plant is also used as cattle fodder to improve milk production. The stem portion of the plant yields the valuable fiber, which has been traditionally used by the tribal for making rope based products used for packing grains and transportation purpose. After extracting the fiber from the stem, residue of the bark portion is used as fuel wood. Traditionally, bark powder is also used as a bandage material for faster healing of wounds and setting of broken bones.
Girardinia heterophylla is among those plant species which have not been systematically studied for their biological active constituents in order to establish their potential use through its different parts viz. leaves, stem and roots. The availability, different uses and phytochemical examination of Girardinia heterophylla(Danskandali) have not been studied in Uttarakhand and scarce literature is available on the plant.
It is of immense importance to screen the medicinal plants growing in nature to find the additional source of bioactive compounds to be used for human welfare. The increasing global interest towards this interface between chemistry and biology has gained more importance and the public demand is continuously rising for the cost effective medications and biological agents from sustainable and natural resources.
The study mainly focuses on the phytochemical examination of species of Girardinia heterophylla grown in Mussoorie hills of Dehradun district of Uttarakhand state.
Material and methods
General experimental procedures
TLC Plates coated with silica gel G to a thickness of 0.25 mm were used. SD-fine silica gel (100-200 mesh) was used for column chromatography. Melting point was determined on a melting point apparatus in a sealed glass capillary tube and mixed melting point (m.m.p.) by authentic sample. GC-MS conditions for analyzing the compounds were TRAC-MS (Finnigancompany).
Plant material
The plant samples were collected from the Middle Himalayas at an altitudinal range of 22,00m to 25,00m in Mussoorie and Dhanaulti areas of Dehradun District. The plant samples of leaves, stem and roots were collected in the month of November 2010.
Extraction and Isolation
The roots (600g) of Girardinia heterophylla were milled after air drying and were extracted with the solvent petroleum ether (60-80oC) and methanol. The petroleum ether extract (2.57%) was obtained and further examined.Petroleum ether extract is column chromatographed over silica gel and elution of the column with varying amount of ethyl acetate in petroleum ether afforded three compounds having code of GHRPTA, GHRPTB and GHRPTC.
Weight of petroleum ether extract = 15.42gm
Weight of silica gel used for adsorption of extract = 75gm
Weight of silica gel used for building of column = 322gm
Solvent used for packing column = Petroleum ether
Retention volume = 435
Volume of each fraction = 100 ml
The further details are given in table-1.
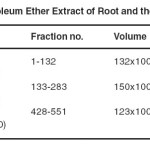 |
Table-1:Compounds of Petroleum Ether Extract of Root and their TLC system with Eluents Click here to View table |
Results and Discussion
Three compounds were isolated from roots of Girardinia heterophylla having code numbers GHRPTA(β-sitosterol), GHRPTB (γ-sitosterol) and GHRPTC (Ursolic acid) are described hereunder.
Compound GHRPTA (β-sitosterol)
It was eluted with petroleum ether: ethyl acetate (98:2) on concentrating yielded white silky needles (45mg, 0.008%, m.p. 136-137oC), [α] D – 100oC (0.05 in CHCl3). It gave Liebermann Burchard test for terpenoids and steroids,having molecular formula C29H50O. (M+, m/z 414). The GC-MS spectrum revealed the compound to be the β-sitosterol [Figure-1,2]. It was identified as β-sitosterol by direct comparison with an authentic sample (m.m.p., Co-TLC and superimposable IR) and by comparison of its mass spectrum (Figure-4A) with that provided in the NIST standard chart library.
β-sitosterol has antidiabetic activity [1]. It reduces the symptoms of Benign Prostatic Hyperplasia (BPH) and also found as an anti-inflammatory agent [2,3].
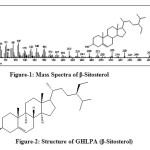 |
Figure-1: Mass Spectra of β-Sitosterol Figure-2: Structure of GHLPA (β-Sitosterol) Click here to View figure |
Compound GHRPTB (γ-sitosterol)
It was eluted with petroleum ether: ethyl acetate (97:3) on concentrating yielded as white crystals (30mg), m.p. 147-148oC, [α]D – 45 (CHCl3). The yield of the γ-sitosterol was found to be 0.010%. It gave Liebermann Burchard test having molecular formula C29H50O, (M+, m/z 414). The compound GHLPB was analyzed by GC-MS. It was identified as γ-sitosterol by comparison of its mass spectra given in figure-3 with that of γ-sitosterol provided in the NIST standard chart library. The mass spectral values correspond to literature [4]. γ-sitosterol has been shown to be an epimer of β-sitosterol. The only difference of these two epimers was the 24-ethyl substituent. The 24-ethyl is present in the side chain of γ-sitosterol [Figure-4]. GC-MS assisted the characterization of γ-sitosterol has been reported in the literature [5-7] has beta-chirality which was indicated as 24S in the NIST standard chart library. This is the first report of isolation of γ-sitosterol in the leaves of Girardinia heterophylla.
γ-sitosterol reduces the hyperglycemia in STZ- induced diabetic rats due to increased insulin secretion and inhibition of glucogenesis. It can be used in Diabetes mellitus [8]. Docking studies of the ligand γ-sitosterol with four different target proteins showed that this is a good molecule which docks well with various targets related to Diabetes mellitus, thus γ-sitosterol can be considered for developing into a protein antidiabetic drug [9].
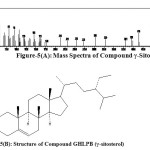 |
Figure-5(A): Mass Spectra of Compound γ-Sitosterol Figure-5(B): Structure of Compound GHLPB (γ-sitosterol) Click here to View figure |
Compound GHRPTC (Ursolic acid)
The fractions were eluted with petroleum ether: ethyl acetate (90:10) on concentrating a white silky needles(8.0mg) was obtained havingm.p. 286oC with 0.001% yield. It was identified as ursolic acid given in [Figure-5,6] and by direct comparison with an authentic sample (m.m.p., Co-TLC and superimposable IR). This is the first report of isolation of ursolic acid [Figure 11(C)] in the plant of Girardinia heterophylla.
Itpossesses diverse pharmacological actions like anticancerous [10, 11] antiulcer [12] hypoglycaemic [13] antihyperlipidemic [14] Antimicrobial [15, 16], antiviral [17], anti-inflammatory [18], antihistaminic [19-21] CNS depressant [22], Hepatoprotective [23], cardiotonic sedative and tonic effects [24].
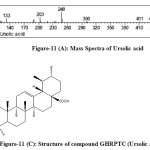 |
Figure-11 (C): Structure of compound GHRPTC (Ursolic acid) Click here to View figure |
Conclusion
The phytochemical constituents were isolated from swollen base of root of Girardinia heterophylla found inMussoorie hills of Uttarakhand. Phytosterols are obtained having 0.008% yield (β-sitosterol), 0.010% yield (γ-sitosterol), and a pentacyclictriterpene acid having 0.001% (Ursolic acid) from roots using petroleum ether extract given in Table-2. In this study the presence of γ-sitosterol and Ursolic acid in roots of Girardinia heterophylla has been reported for the first time.It has been reported to possess many biological activities like antiulcer, anti-diabetic, anti-inflammatory etc.
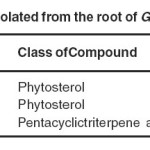 |
List of compounds isolated from the root of Girardinia heterophylla Click here to View table |
Given the attire of identified chemical compounds having potential of being utilized for commercial purposes, the plant can be integrated with region specific developmental activities. Indian Himalayas showcases vast varieties of such plants, many of which are still beyond the ambit of contemporary research. Thus, present study also paves the way for the researchers to carry out further phytochemical investigations in the Himalayan region to identify other plants that may have commercial potential to be used in various industries.
Acknowledgement
The authors gratefully acknowledge Uttarakhand Bamboo and Fiber Development Board (UBFDB) Dehradun for technical guidance
References
- Gupta, R., Sharma, A. K., Dobhal, M. P., Sharma, M. C. and Gupta, R. S. (2011); Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin induced experimental hyperglycemia, Jour. of Diabetes, 3 (29).
- Wilt, T. J., Macdonald, R. and Ishani, A. (1999); Beta-sitosterol for the treatment of benign prostatic hyperplasia (a systematic review). B. J. U. Int., 83 (9), 976.
- Loizou. S., Lekakis, I., Chrousos, G. P. andMoutsatsou, P. (2010); Beta-sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells, Mol. Nut. Food Res., 54, 551.
- Jain, S. P., Bari, B. S. and Surana, J. S. (2010);Isolation of stigmasterol and γ-sitosterol from petroleum ether extract of woody stem of Abelmoschusmanihot,Asian. J. Biol. Sci., 2, 112.
- Piironen, V., Lnsday, G. D. and Miettinen. (2000); Plant sterols: Biosynthesis, biological function and their importance to human nutrition, J. Sci. of Food and Agric., 80, 939.
- Thompson, J. M., Robbins, E. W. and Baker, L. G. (1963); The non homegenity of soybean-gamma sitosterol, J. Steroids, 29 (6), 407.
- Nishihoka , I., Ikekawa, N. and Yagi, A. (1965); Studies on plant sterols and triterpenes, Chem. Pharm. Bull., 11 (5), 579.
- Balamurugan, R., Duraipandiyan, V. and Ignacimuthu, S. (2011); Antidiabetic activity of γ-sitosterol isolated from Lippianodiflora L. in streptozotocin induced diabetic rats, European J. of Pharmacol., 667, 410.
- Balamurugan, R., Stalin, A. and Ignacimuthu, S. (2012); Molecular docking of γ-sitosterol with some target related to diabetes, European J. of Med. Chem., 47, 39.
- Kim, K. W. (1997); Anticancer activities of plant triterpenoids, ursolic acid and oleanoid acid, J. Korean Assoc. Cancer Preven. Metas. and antitumor., 2, 38.
- Young, H. S., Lee, C. K., Park, S. W., Park, K.Y., Kim, K. W., Chung, H. Y., Yokozawa, T. and Oura, H. (1995); Anti-tumorigenic effects of ursolic acid isolated from the leaves of Eriobotrya japonica, Nat. Med., 49, 190.
- Wrzeciono, U., Malecki, I., Budzianowski, J., Kierylowicz, H., Zaprutko, L., Beimvik, E. and Kostepska, H. (1985); Nitrogenous triterpene derivatives, Part 10: Hemisuccunates of some derivatives of oleanolic acid and their antiulcer effects, Pharmaz., 40, 542.
- Ivorra, M. D., Paya, M. and Villar, A. (1989); A Review of Natural Products and Plants as Potentials Antidiabetic Drugs, J. Ethnopharmacol., 27, 243.
- Ma, B. L. (1986); Hyperlipidemic effects of oleanolic acid, Traditional Med. and Pharmacol., 2, 28.
- Collins, M. A. and Charles, H. Y. (1989); Antimicrobial activity of carnosol and ursolic acid: two anti-oxidant constituents of Rosmariusofficinalis, Food Microbiol., 4, 311.
- Newali C. A., Anderson, L. A. and Phillipson, J. D. (1996). Herbal medicine – A Guide for Health Care Professionals, The Pharmaceutical Press, London, 296.
- Kashiwada Y., Wang, H. K., Nagao, T., Kitanaka, S., Yasuda, I., Fujioka, T., Yamagishi, T., Cosentino, L. M., Kozuka, M., Okabe, H., Ikeshiro, Y., Hu, C. Q, Yeh E. and Lee, K. (1998); Anti-AIDS agents, 30 Anti-HIV activity of oleanolic acid, promolic acid and structurally related triterpenoids, J. Nat. Prod., 61 (19), 1090.
- Chattopadhay, D., Arunachalam, G., Mandal, A. B., Bhadra, R., and Mandal, S. C., (2003); CNS activity of MallotuspellatusMurell, Arg, Leaf extract: An ethnomedicine of Bay Islands, J. Ethnopharmacol., 81 (1), 99.
- Tsuruga, T., Chun, Y., Ebizuka, Y. and Sankawa, U. (1991). Biologically active constituents of Melaleucaleucadendron, inhibitors of induced histamine release from rat mast cells. Chem. Pharm. Bull., 39 (12), 3276.
- Wang, B. H., Polya, G. M. (1996); Selective inhibition of cyclic AMP-Dependent protein kinase by amphiphilictriterpenoids and related compounds, Phytochem. Anal., 27, 393.
- Kosuge, T., Yokota, M., Sugiyama, K., Mure, T., Yamazawa, H. and Yamamoto, T. (1985); Studies on bioactive substances in crude drugs used for arthritic disease in trational Chinese medicine-III. Isolation and identification of anti-inflammatory and analgesic principles from the whole herb of PyrolarotundifoliaL, Chem. and Pharm. Bull., 33, 5355.
- Duke, J. A. (1992). Handbook of biologically active phytochemicals and their activities, Boca Raton, F L. CRC Press.
- Shukla, B., Visen, P. K. S., Patnaik, G. K., Tripathi, S. C., Srimal, R. C., Dayal, R. and Dobhal, P. C. (1992); Hepatoprotective activity in the rat of ursolic acid isolated from Eucalyptus Hybrid, Phytother., 6, 74.
- Liu, J. (1995); Pharmacology of oleanolic and ursolic acid, J. Ethnopharmacol., 49 (2), 57.

This work is licensed under a Creative Commons Attribution 4.0 International License.









