NaCN/DOWEX(R)50WX4: A Convenient System for Synthesis of Cyanohydrins from Aldehydes
Sargol Sofighaderi and Davood Setamdideh
Department of Chemistry, Faculty of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, 59135-443, Iran.
DOI : http://dx.doi.org/10.13005/ojc/290340
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
A variety of cyanohydrins were prepared from Aldehydes with sodium cyanide and DOWEX(R)50WX4 in a convenient procedure.
KEYWORDS:Aldehyde;Cyanohydrin;NaCN;DOWEX(R)50WX4
Download this article as:| Copy the following to cite this article: |
| Copy the following to cite this URL: |
Introduction
Cyanohydrins are well-known as important synthetic intermediates1-4. Recently, we have reported that the DOWEX(R)50WX4 (low price cation exchange resin, strong acid) can be used as recyclable catalyst for the regioselective synthesis of Oximes by NH2OH.HCl/DOWEX(R)50WX4 system 5 and reduction of a variety of carbonyl compounds such as aldehydes, ketones, α-diketones, acyloins and α,β-unsaturated carbonyl compounds to their corresponding alcohols by NaBH4/DOWEX(R)50WX4 system 6. In this context, we now wish to report an efficient, facile preparation of cyanohydrins using aldehydes by NaCN/DOWEX(R)50WX4 system in CH3CN at room temperature.
Results and Discussions
The model reaction has been selected by cyanation of benzaldehyde. This reaction was carried out in different solvents, different amounts of the NaCN and DOWEX(R)50WX4for the selection of appropriate conditions at room temperature. Among the tested different solvents, the reaction was most facile and proceeded to give the highest yield in CH3CN. The optimization reaction conditions showed that using 2 molar equivalents of NaCNand 0.5 g of DOWEX(R)50WX4 in CH3CN were the best conditions to complete the cyanation of benzaldehye (1 mmol) to 2-hydroxy-2-phenylacetonitrile (Table 1, Entry 1). Our observation reveals that cyanation completes within 60 min with 95% yields of product as shown in scheme 1.
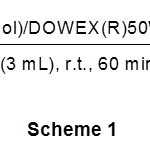 |
Scheme 1 Click here to View figure |
The efficiency of this protocol was further examined by using various structurally different aldehydes. In this approach, the correspondingcyanohydrins were obtained in excellent yields (85-95%) within 60-120 min as shown in Table 1.
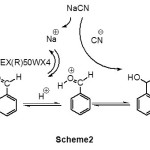 |
Scheme2 Click here to View figure |
The reaction takes place under heterogeneous conditions. The mechanism for the influence of DOWEX(R)50WX4 is not clear, but as shown in scheme 2, we think that with the addition of DOWEX(R)50WX4(as cation exchange resin, strong acid) to the reaction mixture (substrate &NaCN in CH3CN), Na+ with H+ slowly being changed and hydrogen ion concentration increase. Therefore, carbonylgroupcanbeprotonated, thusitis more readilytoattackwith thecyanide anion for the cyanohydrin formation.
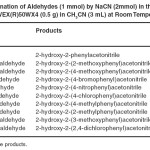 |
TABLE 1:Cyanation of Aldehydes (1 mmol) by NaCN (2mmol) in the presence of DOWEX(R)50WX4 (0.5 g) in CH3CN (3 mL) at Room Temperature. Click here to View table |
Experimental
IR and 1H NMR spectra were recorded on PerkinElmer FT-IR RXI and 400 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples (melting or boiling points). TLC was applied for the purity determination of substrates, products and reaction monitoring over silica gel 60 F254 aluminum sheet.
Cyanation of benzaldehydewith NaCN/DOWEX(R)50WX4, A typical procedure:
In a round-bottomed flask (10 mL) equipped with a magnetic stirrer, a mixture of benzaldehyde (0.106 g, 1 mmol) and DOWEX(R)50WX4(0.5, g) in CH3CN (3 mL) was prepared. The resulting mixture was stirred for 5 min at room temperature. Then the NaCN (0.1 g, 2mmol) was added to the reaction mixture and stirred at room temperature. TLC monitored the progress of the reaction (eluent; CCl4/Ether: 5/2). The reaction was filtered after completion within 60 min. Evaporation of the solvent afforded the 2-hydroxy-2-phenylacetonitrile (0.l26 g, 95% yield, Table 1, entry 1).
Conclusion
We have shown that the NaCN/DOWEX(R)50WX4 is suitable for the cyanation of a variety of aldehydes to their corresponding cyanohydrins in high to excellent yields. Cyanation reactions were carried out with 2 molar equivalents of NaCNin the presence of 0.5 g DOWEX(R)50WX4in CH3CN at room temperature.
Acknowledgements
The authors gratefully appreciated the financial support of this, work by the research council of Islamic Azad University branch of Mahabad.
References
- Gregory, R. J. H. Chem. Rev. 3649 (1999).
- Holmes, I. P. Angew. Chem. Int. Ed. 43: 2752 (2004).
- Chen, F. X.; Feng, X. Synlett 892 (2005).
- Achard, T. R. J.; Clutterbuck, L. A.; North, M. Synlett 1828 (2005).
- Setamdideh, D.; Khezri, B.; Esmaeilzadeh, S. J. Chin. Chem. Soc.59: 1119 (2012).
- Setamdideh, D.; Khezri, B.; Alipouramjad, A. J. Chin. Chem. Soc.60: 590 (2013).

This work is licensed under a Creative Commons Attribution 4.0 International License.









