Design and Synthesis of new Steroid-cyclobutanone derivative
1Lauro Figueroa-Valverde*, 2Francisco Díaz-Cedillo, 3Marcela Rosas-Nexticapa, 4Mendoza-López R,1Elodia García-Cervera, 1Eduardo Pool-Gómez1, 1María López-Ramos
1Laboratorio de Farmacoquímica de la Facultad de Ciencias Químico-Biológicas de la Universidad Autónoma de Campeche. Av. Agustín Melgar, Col Buenavista C.P. 24039, Campeche Cam., México. 2Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional. Prol. Carpio y Plan de Ayala s/n Col. Santo Tomas, México, D.F. C.P. 11340. 3Facultad de Nutrición, Universidad Veracruzana. Médicos y Odontólogos s/n, 91010, Xalapa, Veracruz. México. 4Unidad de Servicios de Apoyo en Resolución Analítica, Universidad Veracruzana. Luis Castelazo Ayala s/n, Col. Industrial Animas, 91190, Xalapa, Veracruz. México.
DOI : http://dx.doi.org/10.13005/ojc/290309
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
In this study, a prednisone derivative was synthetized; the first stage involve the reaction of the compound 1 (11-Bis-(2-amino-ethylimino)-17-[1-(2-amino-ethylimino)-2-hydroxy-ethyl]-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phe- nanthren-17-ol)withchloroacetyl chloride in presence of triethylamineto form2[(3-chloro-2-oxo-cyclobutyl)(1-{3,11-bis-(2-[{2-[(chlorocarbonyl)amino]-ethyl}(3-chloro-2-oxocy-clobutyl)amino]-17-hydroxy-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenantren-17-yl})-2-hydroxyethyl)-amino]ethylcarbamic chloride (3). The second stage was achieved by the reaction of 3 with thiourea in methanol to form the compound 5(2-{(2-Amino-ethyl)-[1-(3,11-bis-[(2-amino-ethyl)-(3-chloro-2-oxo-cyclo- butyl)-amino]-17-hydroxy-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dode-cahydro-3H-cyclopenta[a]phenantren-17-yl)-2-hydroxy-ethyl]-amino}-4-chloro-cyclobuta-none). The structure of compounds obtainedwas confirmed by elemental analysis, spectroscopy and spectrometry data. In conclusion, this method offers some advantages such as good yields, simple procedure, low cost, and ease of workup.
KEYWORDS:Prednisone;thiourea;chloroacetyl chloride;triethylamine
Download this article as:| Copy the following to cite this article: Figueroa-Valverde L, Díaz-Cedillo F, Rosas-Nexticapa M, Mendoza-López R, García-Cervera E, Pool-Gómez E, López-Ramos. Design and Synthesis of new Steroid-cyclobutanone derivative. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290309 |
| Copy the following to cite this URL: Figueroa-Valverde L, Díaz-Cedillo F, Rosas-Nexticapa M, Mendoza-López R, García-Cervera E, Pool-Gómez E, López-Ramos. Design and Synthesis of new Steroid-cyclobutanone derivative. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=274 |
Introduction
Since several years ago, diverse corticosteroid derivatives have been development; for example, the synthesis 3α-acetoxy-16β-methylpregnane-l1,20-dione by catalytically reduction of 3α-acetoxy-16-methy1-16-pregnene-11,20-diene using palladium on charcoal in acetic1. Other reports indicate the preparation of 17α-Hydroxy-3,11,20-triketo-2l-azido-4-pregnene by the reaction of cortisone-21-mesylate with sodium azide in acetone2. In addition, other studies indicate the synthesis of 6α-6β-Dichloro-16α-methylallopregnan-3β-ol-20-one acetate by the reaction of 16α-methyl-D5-pregnen-3β-ol-20-one acetate with carbon tetrachloride in pyridine3. Other studies showed the development of 21-Desoxy-cortisone (7α-Hydroxy-D4-pregnene-3,11,20-trione) by the reaction of cortisone with p-toluenesulfonyl chloride in pyridine4. Additionally, other corticosteroid derivative (Prednisolone 21-[4´-(Bromo)butyrate]) was synthetized by the reaction of prednisone with 4-bromo-butyryl chloride in presence of triethylamine5. Other data indicate the preparation of a prednisone derivative (2-oxo-3-cyano-substituted-androsteno[17,16-c]pyran-3b-ol) by the reaction of prednisolone with ethyl cyanoacetate in the presence of sodium ethoxide6. In addition, a study show the esterification of prednisolone with L-carnitine by the reaction of prednisolone with L-carnitine using 4-dimethylamino-pyridine/N,N´-dicyclo-hexylcarbodiimide7. Also, other report showed the development of prednisolone 21-(4’chlorobenzoate) by the reaction of prednisolone with 4-(chloromethyl)benzoyl chloride and triethylamine8. All these experimental results show several procedures which are available for synthesis of corticosteroid derivatives; nevertheless, expensive reagents and special conditions are required. Therefore, in this study a prednisone derivative was synthetized using some strategies.
EXPERIMENTAL
The compound 1 (11-Bis-(2-amino-ethylimino)-17-[1-(2-amino-ethylimino)-2-hydroxy-ethyl]-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]- phenanthren-17-ol)9 was prepared mainly previously method reported. The other compounds evaluated in this study were purchased from Sigma-Aldrich Co. Ltd. The melting points for the different compounds were determined on an Electrothermal (900 model). Infrared spectra (IR) were recorded using KBr pellets on a Perkin Elmer Lambda 40 spectrometer. 1H and 13C NMR spectra were recorded on a Varian VXR-300/5 FT NMR spectrometer at 300 and 75.4 MHz in CDCl3 using TMS as internal standard. EIMS spectra were obtained with a Finnigan Trace GCPolaris Q. spectrometer. Elementary analysis data were acquired from a Perkin Elmer Ser. II CHNS/0 2400 elemental analyzer.
Synthesis of 2[(3-chloro-2-oxo-cyclobutyl)(1-{3,11-bis-(2-[{2-[(chlorocarbonyl)amino]- ethyl}(3-chloro-2-oxocyclobutyl)amino]-17-hydroxy-10,13-dimethyl-6,7,8,9,10,11,12, 13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenantren-17-yl})-2-hydroxyethyl)-amino]ethylcarbamic chloride.
A solution of compound 1(100 mg, 0.20 mmol), chloroacetyl chloride (64µl, 0.8 mmol) and triethylamine (112µl, 0.8 mmol) in 10 ml of methanol was stirring to reflux for 4 h. The reaction mixture was evaporated to dryness under reduced pressure. The obtained solid was washed with water, yielding 40 % of product, m.p. 196-198oC;IR ṽ = 3402, 1662, 1712, 1198 cm-1; 1H NMR (300 MHz, CDCl3) dH:0.91 (s, 3H), 0.96 (m, 1H), 1.25 (s, 3H), 1.31-1.65 (m, 5H), 1.67 (m, 1H), 1.68 (m, 1H), 1.79-1.93 (m, 4H), 1.97 (m 1H), 1.99 (m, 1H), 2.07 (m, 1H), 2.10-2.25 (m, 3 H), 2.28 (d, 1H), 2.32 (d, 1H), 2.33 (m, 1H), 2,37 (m, 1H), 2,38 (d, 1H), 2.41 (d, 1H), 2.43 (d, 1H), 2.87 (d, 1H), 3.23 (m, 1H), 3.34(t, 2H), 3.37 (t, 2H), 3.40 (m, 1H), 3.61 (t, 1H), 3.63 (m, 1H), 3.67 (t, 1H), 3.81, (m, 1H), 4.22-4.40 (m, 2H), 4.60 (m, 3H), 5.14-5.46 (m, 2H), 5.84 (broad, 5H) ppm. 13 C NMR (75.4 Hz, CDCl3) d: 17.40 (C-24), 21.50 (C-33), 23.40 (C-8), 26.47 (C-17), 31.53 (C-16), 32.27 (C-53), 32.71 (C-40), 32.72 (C-48), 33.68 (C-7), 33.92 (C-3), 35.68 (C-9), 39.40 (C-10), 39.56 (C-36), 40.12 (C-20), 40.16 (C-29), 41.98 (C-5), 49.50 (C-4), 49.70 (C-35), 49.92 (C-28), 50.19 (C-19), 52.12 (C-13), 56. 62 (C-2), 56.70 (C-1), 60.22 (C-45), 62.10 (C-26), 62.96 (C-54, C-42, C-49), 69.50 (C-52), 70.16 (C-47), 71.12 (C-40), 86.66 (C-6), 113.22 (C-12), 135.98 (C-15), 144.80 (C-22, C-31, C-38), 148.86 (C-11), 200.98 (C-55), 201.10 (C-50), 206.10 (C-43) ppm.EI-MS m/z: 982.20 (M+10). Anal. Calcd. for C42H56Cl6N6O8: C, 51.18; H, 5.73; Cl, 21.58; N, 8.53; O, 12.99. Found: C, 51.16; H, 5.70.
Synthesis of 2-{(2-Amino-ethyl)-[1-(3,11-bis-[(2-amino-ethyl)-(3-chloro-2-oxo-cyclo- butyl)-amino]-17-hydroxy-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahy- dro-3H-cyclopenta[a]phenantren-17-yl)-2-hydroxy-ethyl]-amino}-4-chloro-cyclobuta-none
A solution of compound 3 (100 mg, 0.10 mmol), thiourea (50 mg, 0.66 mmol) in 10 ml of methanol was stirring for 72 h to room temperature. The reaction mixture was evaporated to dryness under reduced pressure. The obtained solid was washed with aqueous sodium thiosulfate to remove excess iodine and then washed with water, yielding 56% of product, m.p. 136oC;IR ṽ = 3398, 3380, 1714, 1196 cm-1; 1H NMR (300 MHz, CDCl3) dH: 0.91 (s, 3H), 0.97 (m, 1H), 1.11 (s, 3H), 1.22 1.62 (m, 5H), 1.69 (m, 1H), 1.70 (m, 1H), 1.71-1.77 (m, 2H), 1.84 (broad, 8H), 1.86-1.96 (m, 2H), 1.97 (m, 1H), 1.98 (m, 1H), 2.07(m, 1H), 2.10-2.25 (m, 3H), 2.35 (m, 1H), 2.59 (t, 2H), 2.62 (t, 2H), 2.65-2.68 (t, 2H), 2.70 (t, 2H), 2.71 (t, 2H), 2.76 (t, 2H), 2.91 (m, 1H), 3.23 (m, 1H), 3.40 (m, 1H), 3.62 (t, 1H), 3.65 (m, 1H), 3.72 (t, 1H), 3.82 (m, 1H), 4.25-4.41 (m, 2H), 4.63 (m, 3H), 5.14-5.47 (m, 2H) ppm. 13 C NMR (75.4 Hz, CDCl3) d:17.10 (C-22), 21.50 (C-29), 23.40 (C-8), 26.47 (C-17), 31.78 (C-16), 32.24 (C-45), 32.70 (C-35), 32.72 C-41), 33.68 (C-7), 33.90 (C-3), 35.68 (C-9), 39.40 (C-10), 42.12 (C-5), 42.96 (C-32), 47.12 (C-20), 47.16 (C-27), 49.50 (C-4), 50.28 (C-26), 50.52 (C-50.30), 52.95 (C-31), 56.65 (C-2), 56.71 (C-1), 60.22 (C-38), 62.17 (C-24), 62.96 (C-36, C-42, C-46), 69.52 (C-44), 70.16 (C-40), 71.02 (C-34), 86.46 (C-6), 113.16 (C-12), 121.68 (C-14), 136.30 (C-15), 148.80 (C-11), 201.36 (C-47), 201. 76 (C-43), 202.02 (C-37) ppm.EI-MS m/z: 796.36 (M+10). Anal.Calcd.for C39H59Cl3N6O5: C, 58.68; H, 7.45; Cl, 13.32; N, 10.53; O, 10.02. Found:C, 56.64; H, 7.44.
Results and Discussion
There are many procedures for development of corticosterids derivatives. Nevertheless, despite its wide scope, have some drawbacks e.g., several agents used have a limited stability and their preparation require condition specials10,12. Analyzing these data, in this study we report a straightforward route for synthesis of a prednisone derivative (5) using some strategies. The first stage step was achieved by the reaction of 1 with chloroacetyl chloride to form a chloroamide derivative involved in compound 3. It is important to mention that there are many procedures for the formation of chloroamides are known in the literature, for example the reaction of amine with Trichloroisocyanuric Acid13 or amide secondary with N-chlorobenzotriazole to form a chloroamide derivative14. In addition, since several years ago, have been prepared some chloroamides using chloroacetyl chloride15,16. Analyzing these data, in this study chloroacetyl chloride was used to form a chloroamide involved in the compound 3. The 1H NMR spectrum of compound 3 shows signals at 0.91 and 1.25 ppm for methyl groups; at 0.96, 1.31-1.65, 1.79-1.93, 2.10-2.25, 3.23, 4.22-4.40 5.14-5.46 ppm for steroid nucleus; at 1.67-2.07, 2.33, 3.40, 3.81 and 4.60 ppm for protons of ciclobutane rings; at 2.37, 243 and 3.34ppm for arm bound to C-ring of steroid nucleus; at 2.28, 2.238 and 3.33 ppm for arm bound to B-ring of steroid nucleus; at 2.32, 2.41 and 3.37 ppm for arm bound to A-ring of steroid nucleus. Finally, other signals at 2.87 ppm for methylene group bound to both amino and cyclopentane groups; at 5.84 ppm for both chloroamide and hydroxyl groups; at 3.63 and 3.67ppm for methylene group bound to hydroxyl group were found. The 13C NMR spectrum contains peaks at 17.40 and 21.50 ppm for methyl groups; at 23.40-31.53, 33.68-39.40, 41.98-49.50, and 52.10-56.70, 86.66-135.98 and 148.86 ppm for steroid nucleus; at 32.27-32.72 and 62.50-71.12 ppm for carbons involved in cyclobutane rings; at 39.56, 49.70 ppm for methylene involved in the arm bound to A-ring of steroid nucleus; at 40.12 and 50.19 ppm for carbons involved in the arm bound to C-ring of steroid nucleus; at 40.16 and 49.92 ppm for methylene groups involved in the arm bound to D-ring of steroid nucleus; at 60.22 ppm for methylene group bound to hydroxyl group; at 60.10 ppm for carbon bound to both amino and cyclopentene groups. Finally, other signals at 144.80 ppm for chloroamide groups; at 200.98-206.10 ppm for cyclobutanone groups were found.In addition the presence of compound 3was further confirmed from mass spectrum which showed a molecular ion at m/z 982.20.
The second stage was achieved by the removal of chloroacetyl group involved in the compound 3. It is important to mention that, there are some methods that show the removal of chloroacetyl groups of some compounds17; for example, the reaction of chloroacetylamine with thiourea to form an amine derivative18,19. In this study, for the removal of chloroacetyl group was used thiourea to form a primary amine involved in the compound 5. This reaction is carried out an intramolecular amidinolysis to release the corresponding amine.The 1H NMR spectrum of compound 5 shows signals at 0.91 and 1.11 ppm for methyl groups; at 0.97, 1.21-1.62, 1.71-1.77, 1.86-1.96, 2.10-2.25, 3.23, 3.65, 4.25-4.41 and 5.14-5.47 ppm for steroid nucleus; at 1.69, 1.70, 1.97, 1.98-2.07, 2.35, 3.40, 3.82 and 4.63 ppm for protons involved in cyclobutane rings; at 2.62 and 2.76 ppm for methylene groups involved in the arm bound to A-ring of steroid nucleus; at 2.59 and 2.71 ppm for protons involved in the arm bound to C-ring of steroid nucleus; at 2.65-2.70 ppm for methylene groups involved in the arm bound to D-ring of steroid nucleus. Finally, other signals at 1.84 ppm for both hydroxyl and amino groups; at 2.91 ppm for methylene group bound to both amino and cyclopentene groups; at 3.62 and 3.72 ppm for methylene group bound to hydroxyl group were found. The 13C NMR spectrum contains peaks at 17.10 and 21.50 ppm for methyl groups; at 23.40-31.78, 33.68-42.12, 49.50, 56.65-56.71 and 86.46-148.80 ppm for steroid nucleus; at 32.24-32.72 and 62.96-71.02 pm for carbons involved in cyclobutane groups; at 42.96 and 52.95 ppm for methylenes of arm bound to A-ring of steroid nucleus; at 47.12 and 50.52 ppm for arm bound to C-ring of steroid nucleus; at 47.16 and 50.28 ppm for methylenes of arm bound to D-ring of steroid nucleus. Finally, other signals at 60.22 ppm for methylene bound to hydroxyl group; at 62.17 ppm for carbon bound to both amino and cyclopentene groups; at 201.36-202.02 ppm for cyclobutanone groups were found. In addition, the presence of compound 5was further confirmed from mass spectrum which showed a molecular ion at m/z 796.36
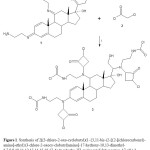 |
Figure 1. Synthesis of 2[(3-chloro-2-oxo-cyclobutyl)(1-{3,11-bis-(2-[{2-[(chlorocarbonyl)- amino]-ethyl}(3-chloro-2-oxocy-clobutyl)amino]-17-hydroxy-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenantren-17-yl})-2-hydroxyethyl)-amino]ethylcarbamic chloride (3). Reaction of the compound 1 (11-Bis-(2-amino-ethyl- imino)-17-[1-(2-amino-ethylimino)-2-hydroxy-ethyl]-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phe- nanthren-17-ol)withchloroacetyl chloride to form 3. i = triethylamine Click here to View figure |
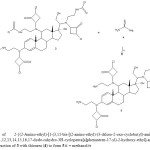 |
Figure 2.Synthesis of 2-{(2-Amino-ethyl)-[1-(3,11-bis-[(2-amino-ethyl)-(3-chloro-2-oxo-cyclobutyl)-amino]-17-hydroxy-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dode-cahydro-3H-cyclopenta[a]phenantren-17-yl)-2-hydroxy-ethyl]-amino}-4-chloro-cyclobuta-none (5). Reaction of 3 with thiourea (4) to form 5.ii = methanol/rt Click here to View table |
Conclusions
In this study, we reported an efficient and simple method for synthesis of a steroid-cyclobutanone derivative. It is important to mention that this method is highly versatile and the yield is good.
References
- Rausser R., Lyncheski A., Harris H., Grocela R., Murrill N., Bellamy E., Ferchinger D., Gebert W., Herzog H., Hershberg E. andOliveto E.,J. Org. Chem., 31:26-31 (1966).
- Brown H., Matzuk R., Hoff D. and Sarett H., J. Org. Chem., 26:5052-5054 (1961).
- Batres E., Cardenas T., Edwards J., Monroy G., Mancera O., Djerassi C. andRingold H., J. Org. Chem., 26:871-878 (1961).
- Bowers A. and Ringold H. J. Am. Chem. Soc., 80:3091-3093 (1958).
- Baraldi P, Romagnoli R, Nuñez, PerrettiM,Clark P, Ferrario M, Govoni M, Benedini F. andOngini E.,J. Med. Chem. 47:711-719 (2004).
- GalilA. and Abdulla M. Archiv. Der Pharm., 339:88-95 (2006).
- Mo J, Shi S,Zhang Q, Gong T, Sun X. and Zhang Z. Mol. Pharm., 8:1629-1640 (2011).
- Clark M, Del Soldato P,Fiorucci S, Flower R. andPerretti.British J.Pharmacol.,131:1345-1354 (2000).
- Figueroa-Valverde L, Díaz-Cedillo F, García-Cervera E, 1Pool Gómez E, Rosas-Nexticapa M, Mendoza-López R, López-Ramos M. and May-Gil I.Asian J. Chem., 2013. In press.
- Oh S. and Monder C.,J. Org. Chem.,41:2477-2480 (1976).
- Han A. and Monder C. J. Org. Chem., 47:1580-1584 (1982).
- Erlanger B, Borek F, Beiser S. and Lieberman S. J. Biological Chem., 234:1090-1094 (1959).
- Hiegel G, HogenauerT. and C. Lewis, Syn. Com.,35: 2099-2105 (2005).
- Katritzky A, Majumder S and Jain R.Arkivoc., xii:74-79(2003).
- Harte A. and Gunnlaugsson T.Tet. Lett.,47:6321-6324 (2006).
- Katke S, Amrutkar S, BhorR. and Khairnar M.Int. J. Pharm. Sci. Res., 2:148-156 (2011).
- SteglichW. and Batz H.Angew. Chem. Intern., 10:75-76 (1971).
- Masaki M, Kitahara T, KuritaH. and Ohta M.J. Am. Chem. Soc., 90:4508-4509 (1968).
- BertoliniM. and Glaudemans C.Carbohydrate Res.,15:263-270 (1970).

This work is licensed under a Creative Commons Attribution 4.0 International License.









