Cu (II) extraction from sulfate media by functionalized Algerian bentonites
Djamila Bouazza1*, Abdelkader Tayeb1, Mohamed Sassi1, Abdelkader Bengueddach1 and Anne Boos2
1Laboratoire de Chimie des Matériaux, Département de Chimie, Faculté des Sciences -Université d’Oran, El Menaouar B.P 1524 Oran31000.Algeria. 2Institut Pluridisciplinaire Hubert Curien, UMR 7178, CNRS - ECPM - Université de Strasbourg, 25 rue Becquerel, 67087 Strasbourg Cedex, France
DOI : http://dx.doi.org/10.13005/ojc/290319
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
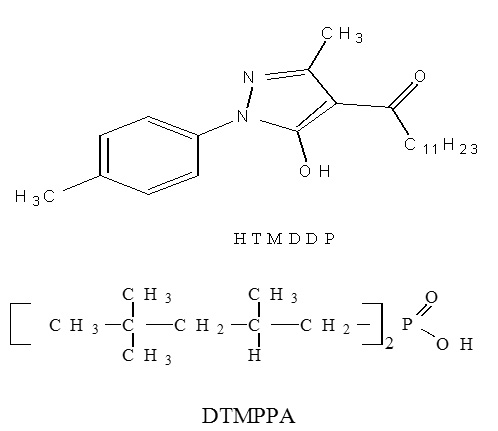
The solid-liquid extraction of Cu(II) from sulfate media at 25°C by sodium homoionic montmorillonites impregnated either with di-2,4,4-trimethylpentyl phosphinic acid(DTMPPA) whose commercial name is Cyanex 272, or with1-tolyl-3-methyl-4-dodecanoylpyrazol-5-one (HTMDDP) has been studied. The extracting agent impregnated solids were characterized by infrared spectroscopy (IR) and X-ray diffraction. The total amounts of loaded extractants were determined by UV spectrometry on washings and/or parent solutions. The ability of each impregnated clay to remove copper from aqueous media was studied in terms of shaking time, equilibrium pH and solid/liquid ratio. The different experimental results have shown that the clay containing HTMDDP leads to better performances for the extraction of copper with respect to capacity as well as pH values. The capacities for HTMDDP and DTMPPA impregnated solids are found to be 320 mmol/kg and 200 mmol/kg and extraction is total at equilibrium pH above 2.2 and 4.7, respectively. The HTMDD-impregnated clay has been found to have high metal ion extractability and hence good potential as an alternative to established solvent extraction systems
KEYWORDS:Bentonite;clay – impregnation;Solid-liquid extraction;Copper;Separation
Download this article as:| Copy the following to cite this article: Bouazza D, Tayeb A, Sassi M, Bengueddach A, Boos A. Cu (II) extraction from sulfate media by functionalized Algerian bentonites. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290319 |
| Copy the following to cite this URL: Bouazza D, Tayeb A, Sassi M, Bengueddach A, Boos A. Cu (II) extraction from sulfate media by functionalized Algerian bentonites. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=292 |
INTRODUCTION
Over the last few years, the pollution due to heavy metals has reached critical levels. It may often end up in ground and surface water short-term poisoning because of its occurrence in aqueous waste streams and soils surrounding many industrial areas. Consequently, the development of advanced methods that can combine high efficiency to remove heavy metal pollutants from waste streams and low cost is needed. In this context, abundant and inexpensive materials such as clays and clay minerals are often implicated. Several substances such as kaolin, montmorillonite and illite are commonly used in order to remove lead, copper, zinc and cadmium from acid aqueous solutions [1-10]. However, if all these materials show a certain activity, the results obtained remain below expectations. Several studies dealing with the sorption of heavy metals on extractant-loaded solid supports have been performed during the last decade with the aim to improve the sorption capacity [11-15]. Surface modification of the latter was shown to be an important feature for developing their practical applications such as fillers and adsorbents. This was obtained by means of intercalation reactions through interlamellar grafting of organic molecules [16-26]. Although organophosphorous and pyrazolone chelating extractants are often used in the liquid-solid extraction process of metals [11, 17 – 18, 27 – 38], any investigation concerning clays functionalized by pyrazolones is not described in the literature. In this work, the solvent extraction reagents DTMPPA and HTMDDP are introduced by the dry impregnation method into a montmorillonite clay previously treated. The obtained sorbents are characterized by infrared spectroscopy, chemical analysis and X-ray diffraction and their behaviors with respect to the extraction of copper from dilute sulfate solutions are compared.
EXPERIMENTAL
Materials and Reagents
Natural montmorillonite clay (natural clay) was supplied by ENOF Corp., Algeria. DTMPPA was provided by CYTEC as Cyanex 272 and used after purification [39]. HTMDDP was supplied by the SNPE Corp., France. Stock solutions of Cu (II) (1 g/L) were prepared by dissolving the corresponding salt (CuSO4, Merck a.r. grade) in deionised water. Products used for the preparation of the different solutions such as Na2SO4, NaCl, HCl, H2SO4, ethanol and toluene were all Merck a. r. grade.
Preparation of impregnated sorbents
First, the natural clay was purified with a hydrochloric acid solution according to the following protocol: a mixture of clay (10 g) with an aqueous chlorhydric acid solution (500 ml, 2M) was prepared and stirred up for 24h at 25°C; the solid was then separated by centrifugation and washed with distilled water in order to eliminate the cationic impurities present in the natural clay. The clay structure constancy was checked by DRX. Second, the substrate was added to an aqueous sodium chloride solution (500 ml, 0.5 M) and the obtained suspension was stirred up for 4 h at 70 °C. The solid obtained was subsequently separated by centrifugation. This treatment was repeated four times. Consequently, the homoionic clay obtained was washed thoroughly with distilled water to remove excess NaCl, dried at 80 °C for 24 h and ground to pass 63 µm mesh sieve. DTMPPA and HTMDDP were introduced into the solid clay supports by means of the dry impregnation method already successfully used [20]. An appropriate amount of homoionic clay (1g) was mixed with 10 mL of an ethanolic solution containing 1 mmol (0.29 g) of DTMPPA, or with 10 mL of a toluene solution containing 1mmol (0,358 g) of HTMDDP. In both cases, the mixture was continuously stirred up under atmospheric pressure until total solvent evaporation. The obtained solid was washed with HNO3 0.5 M, to avoid the ligand dissolution in the aqueous phase in the form of its anion A–, then dried under atmospheric pressure at 80°C for 24 h and finally ground to pass 63µm mesh sieve.
The two impregnated materials obtained are named HTMDDP@clay and DTMPPA@clay.
Methods of characterization
The chemical compositions of the starting materials (natural and homoionic clays) were determined following their dissolution in aqua regia solution, removal of the insoluble silica by filtration and analysis with atomic emission spectrometry (ICP-AES Varian Liberty 220). Silica content was determined by gravimetric analysis of the insoluble residue using hydrofluoric acid to form volatile silicon tetrafluoride. FTIR Attenuated Total Reflectance spectra (4000–400 cm-1) of the solids were recorded with a Spectrum One Perkin Elmer instrument. The XRD powder patterns were performed with CuKα1 radiation (1.5406 Ǻ) on a Siemens D5000 diffractometer. The mass of loaded HTMDDP or DTMPPA was quantitatively determined by UV spectrometry (HP 8453 Hewlett Packard) on the washing solutions and the mass-balance of the extractant.
2.4 Metal extraction procedure
The extraction of Cu (II) (100 mg/L, 1.57 mM) was carried out in polypropylene tubes. 0.1 g of impregnated solid was mixed mechanically for 150 min with 10 mL of Cu (II) 100 mg/L at (25 ± 0.2)°C. The ionic strength of the solution was maintained constant by addition of 0.33 M (H+,Na+)SO42- solution. The phases were then separated by high speed centrifugation (12000 rpm for 10 min) and the liquid phase pH (equilibrium pH: pHe) was measured. Copper contents in the aqueous phases were determined by atomic absorption spectroscopy on a Perkin–Elmer 2380 spectrophotometer using an air acetylene flame.
RESULTS AND DISCUSSION
Characterization of the solids
The chemical compositions of natural and homoionic clays are reported in Table 1. The acid treatment followed by the sodium exchange allowed the partial elimination of Fe, Mg and Ca elements which degrade the extraction yields by complexation of the loaded ligands in the impregnated solids as shown in figure 4.
The DTMPPA and HTMDDP contents of the impregnated solid supports are quite similar: we found 720 mmol.kg-1 and 730 mmol.kg-1, respectively. This result allows a direct comparison of the two solids efficiencies.
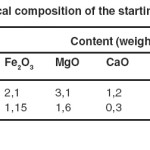 |
Table 1: Chemical composition of the starting clay materials Click here to View table |
IR analysis
FTIR spectra of the homoionic clay and impregnated solids are illustrated in figure 1. The IR spectrum of homoionic clay shows the presence of absorption bands of the clay phase and the characteristic absorption bands of impurities such as quartz and calcite. The bands observed between 3200 and 3600 cm-1 correspond to both the OH stretching vibration of adsorbed water and the silicated skeleton; the peak at 1650 cm-1 is also attributed to adsorbed water. Other impurities such as quartz are difficult to identify since the main Si-O vibration band of montmorillonite appearing at 1050 cm-1 is wide enough to mask the corresponding band of free silica in quartz which ranges from 900 cm-1 to 1200 cm-1. Another band located at 1394 cm-1 characterizes calcite.
Besides the absorption bands characterizing montmorillonite, the IR spectrum of DTMPPA@clay shows the characteristic vibration bands of DTMPPA between 2970 cm-1 and 2890 cm-1 assigned to the stretching modes of the methyl groups and aliphatic C—H groups 11 and near 1380 cm-1 assigned to the phosphoryle stretching 40. In the case of HTMDDP@clay, the IR spectrum shows the presence of the main vibration bands characteristic of HTMDDP between 2960 cm-1 and 2870 cm-1 assigned to the stretching modes of aliphatic groups 41. These results confirm the presence of DTMPPA and HTMDDP in the corresponding solids.
 |
Figure 1: FTIR spectra homoionic clay (a), DTMPPA@clay (b) and HMDDP@clay (c). Click here to View figure |
X-ray diffraction
Figure 2 presents the XRD powder pattern of natural and homoionic clays. The former is characteristic of montmorillonite and is in good agreement with earlier works 42. This mineral contains also an impurity identified as quartz characterized by a strong reflection at 2θ = 26.6° in agreement with the IR results.
The XRD pattern of homoionic clay shows a decrease in the basal spacing from 14.53 to 12.03 Ǻ. This behavior is probably due to the cation exchange of interlayer hydrated metallic ions by sodium cations.
Figure 2 also shows the XRD powder patterns of DTMPPA@clay and HTMDDP@clay. The noticeable modification with respect to the ligand free clay pattern is observed on the (001) reflection. It is moved toward lower angles for both impregnated solids due to an enlargement of the interlayer space from 12.30 Å to about 14.30 Å. In both cases, the increase in the interfoliar distance is probably due to the intercalation of the organic ligand between the sheets. Some reflections from the HTMDDP crystallized ligand are clearly visible at 2q = 5.9° (15 Ǻ) and 8.4° (10.5 Ǻ). Surprisingly, new reflections may be observed on the XRD pattern of the DTMPPA@clay sample in particular at 2q = 5.6° and 6.15°. It is possible that the confinement of the DTMPPA ligand in the interlayers induces its crystallization.
 |
Figure 2: Powder XRD pattern (Cu K1) homoionic clay (a), HTMDDP ligand (b), HTMDDP@clay (c) and DTMPPA@clay (d). Click here to View figure |
Metal extraction
The ability of impregnated-clays to adsorb copper metal ions from aqueous solutions was studied by varying different parameters namely the contact time, the pH and the amount of solids.
The solid-liquid extraction of a metal ion may be described by the following equilibrium:
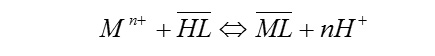
where the highlighted species are in the solid phase. The extraction percentage of a metal may be expressed as:
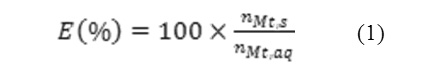
where nMt,s and nMt,aq denote the total mole number of metal in the solid phase at equilibrium and the initial total mole number of metal in the aqueous phase, respectively; “total” means “under any chemical form”. This percentage may also be determined from the analytical concentrations of the metal in the aqueous phase.
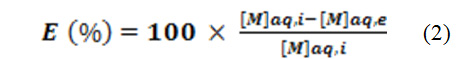
where [M]aq,i and [M]aq,e are the concentrations in the aqueous phase before and after extraction, respectively.
Effect of contact time
The effect of the shaking contact time was studied for an initial pH (pHi) fixed at 4.3 for HTMDDP@clay and 4.8 for DTMPPA@clay solids. Figure 3 shows the evolution of extraction versus shaking time. The extraction reaches a plateau within 30 minutes for homoionic clay and DTMPPA@clay whereas HTMDDP@clay extracts 100% of the available copper after 60 minutes of contact. To check that the observed plateau is not due to the absence of free ions in solution, an experiment with a higher copper content (1000 mg/L) was realized. This experiment confirmed that 60 minutes were sufficient to reach the adsorption equilibrium. For the remainder of the study, the shaking time was set up to 150 minutes.
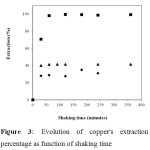 |
Figure 3: Evolution of copper’s extraction percentage as function of shaking time Click here to View figure |
Effect of equilibrium pH
The pH of the aqueous solutions is an important variable for the extraction of metals on the adsorbents, especially with these H+ exchanger ligands. The metal extraction by homoionic and impregnated montmorillonite clays was studied as a function of pH in the limited range from 1 to 5 in order to avoid hydroxide precipitation occurring at higher pH values. Figure 4 shows the measured copper extraction percentage as a function of pHe.
The lowest Cu2+ sorption percentage is obtained in the case of the homoionic clay. The Na+ cations are merely exchanged with Cu2+ cations contained in the aqueous solutions. The highest adsorption rates are observed with the impregnated clay samples because of the presence of the organic ligands. However, HTMDDP@clay displays noticeably higher capacity than DTMPPA@clay: 100% adsorption against only 40%. This fact may be explained by the thorough availability of each HTMDDP molecule in the solid to the contrary of the partial use in the case of DTMPPA ligand. It should be noticed that neither XRD nor FTIR spectroscopy is suited to reveal any differences in the ligand setting in the clay. It could be assumed that the interaction between the ligand and the clay let the complexing head more accessible to metal ions in the case of HTMDDP than DTMPPA. With the latter ligand, we could also suspect the formation of aggregates in the solid. This was generally observed in organic solvents for organo phosphinic acids. The species extracted are generally assumed to be ML2(HL)x with x = 2 or 3 40. With this assumption, 4 or 5 ligand molecules are necessary to achieve the complexation. On the contrary, the formation of more simple species (ML2) is generally observed with the b-dicetone ligand family to which HTMDDP belongs.
Figure 4 shows that the extraction of copper by HTMDDP impregnated solid is total when the pHe value is equal to 2.2. On the other hand, the maximum amount of copper extracted by the solid impregnated with DTMPPA is only 41% and this at a pHe above 4.7. In liquid-liquid extraction, copper ions in sulfate medium are extracted by DTMPPA in the same pH range 43. It appears that if the acylpyrazolones such as HTMDDP are efficient to extract copper in the clay, the organophosphorus acids such as DTMPPA are not. However, previous studies showed that immobilized DTMPPA is efficient for zinc extraction whatever the nature of the solid ( Levextrel or Amberlite XAD-2 resins [44-46], sol-gel 47or mesoporous silica 48, clays 20, and the extraction method 49. Thus, the present DTMPPA impregnated solid may be envisaged for continuous separation of zinc and copper from their dilute sulfate solutions.
As observed in liquid-liquid extraction for a similar ligand, 1-phenyl-3-methyl-4-stearoyl-5-hydroxypyrazole, noted HPMSP, the b-dicetone ligand HTMDDP allows the copper extraction at lower pH than DTMPPA. Furthermore, for HPMSP, the extraction pH is lowered to 2 when the extraction occurs with a solid support like mesostructured silica, but only to 4 when the extraction is performed with an organic solvent 37. With this ligand, the confinement imposed might lead to a decrease in the distance between the two complexing heads required for the formation of the stable CuL2 complex, enhancing the extraction. We have observed the same phenomenon with HTMDDP immobilized in clay.
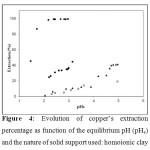 |
Figure 4: Evolution of copper’s extraction percentage as function of the equilibrium pH (pHe) and the nature of solid support used: homoionic clay Click here to View figure |
Effect of the mass of the solid
The effect of the solid mass was studied by varying the amount of the impregnated solids from 0.05 to 0.5 g. The initial pH, pHi, was fixed at 2.6 and 4.54 for the HTMDDP and DTMPPA impregnated solids, respectively. Figure 5 shows that the variation of the adsorption percentage of metal ions as a function of the impregnated solid mass is linear. An extraction maximum (~100%) was obtained with a mass of HTMDDP@clay equal to 0.1 g. When using the DTMPPA@clay, 0.3 g is needed for a 100% extraction yield.
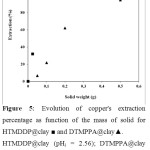 |
Figure 5: Evolution of copper’s extraction percentage as function of the mass of solid for …. Click here to View figure |
Copper loading capacities
Copper extraction capacities of the impregnated montmorillonite clays were studied. The results are given in figure 6. A capacity of 320 mmol/kg is obtained for HTMDDP@clay. This capacity is higher than the DTMPPA@clay one whose value is 200 mmol/kg. The ligand/metal ratio Ls/Ms is calculated with Ls the total number of free or coordinated “HTMDDP” or “DTMPPA” sites and Ms the total number of copper ions in the solid. Ls/Ms ratio is equal to 2 for HTMDDP and corresponds to the formation of CuL2 complex. For DTMPPA, this ratio is equal to 3.5. This is probably due to the formation of a mixture of species such as ML2(HL)x with x = 1 or 2.. The immobilization of the phosphorous ligand in the clay can limit its movement to form the higher level aggregates with x = 2 or 3 as observed in liquid-liquid extraction.
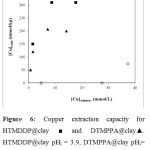 |
Figure 6: Copper extraction capacity for HTMDDP@clay….. Click here to View figure |
CONCLUSION
In this paper, montmorillonite homoionic clay is successfully loaded with either DTMPPA or HTMDDP by the dry impregnation method. It is shown that the two impregnated solids can be profitably used for the extraction of copper from dilute aqueous solutions. However, HTMDDP loaded clay displays a better performance than the phosphorous ligand loaded clay for the adsorption of copper. Indeed, the extraction of copper by the former is complete even at a pHe value as low as to 2.2. This fact may be advantageously exploited for the treatment of acidic effluents. This study also demonstrates a full accessibility of the HTMDDP molecules impregnated in the homoionic clay to the metal ions. The dependence of the extraction on pH implies that the extraction mechanism of the metal ions proceeds by exchange of the acylpyrazolone protons. The formation of CuL2 complex is observed in the case of HTMDDP but more complex stoechiometries are obtained with DTMPPA.
ACKNOWLEDGMENTS
We acknowledge Pr. D. Bendedouch for her help.
REFERENCES
- Farrah H, Pickering W.F. pH effects in the adsorption of heavy metal ions by clays. Chemical Geology., 25, 317 (1979).
- Farrah H, Hatton D, Pickering W.F. The affinity of metal ions for clay surfaces. Chemical Geology 28, 55 (1980).
- Eold J, Pickering W.F. Influence of electrolytes on metal ion sorption by clays. Chemical Chemical Geology., 33, 91 (1981).
- Lacin O, Bayrak B, Korkut O, Sayan E. Modeling of adsorption and ultrasonic desorption of cadmium(II) and zinc(II) on local bentonite. Journal of Colloid and Interface Science. 292, 330 (2005).
- Mellah A, Chegrouche S. The removal of zinc from aqueous solutions by natural bentonite.Water research., 31,621 (1997).
- Veli S, Alyuz B. Adsorption of copper and zinc from aqueous solutions by using natural clay. Journal of Hazardous Materials. 149, 226 (2007).
- Tahir S.S, Rauf N. Removal of Fe(II) from the wastewater of a galvanized pipe manufacturing industry by adsorption onto bentonite clay. Journal of Environmental Management. 73, 285 (2004).
- Yavuz O, Altunkaynak Y, Guzel F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water research. 37, 948 (2003).
- Suraj G, Iyer C.S.P, Lalithambika M. Adsorption of cadmium and copper by modified kaolinites. Applied Clay Science., 13, 293 (1998).
- Gupta S.S, Bhattacharyya K.G. Removal of Cd(II) from aqueous solution by kaolinite, montmorillonite and their poly(oxo zirconium) and tetrabutylammonium derivatives. Journal of Hazardous Materials. 128, 247 (2006).
- Cortina J.L, Miralles N, Sastre A, Aguilar M, Profumo A, Pesavento M. Solvent-impregnated resins containing di-(2,4,4-trimethylpentyl) phosphonic acid I. Comparative study of di-(2,4,4-trimethylpentyl)phosphinic acid adsorbed into Amberlite XAD-2 and dissolved in toluene. Reactive Polymers. 21, 89 (1993).
- Perez M.R, Pavlovic I, Barriga C, Cornejo J, Hermosn M.C, Ulibarri M.A. Uptake of Cu2+, Cd2+ and Pb2+ on Zn-Al layered double hydroxide intercalated with edta. Applied Clay Science.32, 245 (2006).
- Kameda T, Takeuchi H, Yoshioka T. Uptake of heavy metal ions from aqueous solution using Mg-Al layered double hydroxides intercalated with citrate, malate, and tartrate. Separation and Purification Technology 62, 330 (2008).
- Kameda T, Takeuchi H, Yoshioka T. Kinetics of uptake of Cu2+ and Cd2+ by Mg-Al layered double hydroxides intercalated with citrate, malate, and tartrate. Colloids and Surfaces A: Physicochemical and Engineering Aspects 355, 172 (2010).
- Wagner N, Carvalho C. V, Fontana J. Ni(II) removal from aqueous effluents by silylated clays. Journal of hazardous materials 153, 1240 (2008).
- Mercier L, Pinnavaia T.J. A functionalized porous clay heterostructure for heavy metal ion (Hg2+) trapping. Microporous and Mesoporous Materials 20, 101 (1998).
- Cox M, Rus-Romero J.R, Sheriff T.S The application of montmorillonite clays impregnated with organic extractants for the removal of metals from aqueous solution: Part I. The preparation of clays impregnated with di-(2-ethylhexyl) phosphoric acid and their use for the removal of copper(II). Chemical Engineering Journal 84, 107 (2001).
- Cox M, Rus-Romero J.R, Sheriff T.S. The application of monmorillonite clays impregnated with organic extractants for the removal of metals from aqueous solution. Part II. The preparation of clays impregnated with commercial solvent extraction reagents and their use for the removal of copper(II). Reactive and Functional Polymers 60, 215 (2004).
- Erdemoglu M, Erdemoglu S, SayIlkan F, Akarsu M, Sener S, Sayilkan H. Organo-functional modified pyrophyllite: preparation, characterisation and Pb(II) ion adsorption property. Applied Clay Science 27, 41 (2004).
- Bouazza D, Miloudi H, Sassi M, Boos A, Goetz G, Tayeb A, Bengueddach A. Preparation of montmorillonite clays containing DTMPPA for Zinc extraction. Journal of Physics and Chemistry of Solids 67, 1032 (2006).
- Carvalho V, Vignado C, Fontana J. Ni(II) removal from aqueous effluents by silylated clays. Journal of Hazardous Materials 153, 1240 (2008).
- A.de Mello Ferreira Guimaraes A, Ciminelli V.S, Vasconcelos W.L Smectite organofunctionalized with thiol groups for adsorption of heavy metal ions. Applied Clay Science 42, 410 (2009).
- Jiang M.Q, Wang Q.P, Jin X.Y, Chen Z.L. Removal of Pb(II) from aqueous solution using modified and unmodified kaolinite clay. Journal of Hazardous Materials 170, 332 (2009).
- Mellouk S, Cherifi S, Sassi M, Marouf-Khelifa K, Bengueddach A, Schott J, Khelifa A. Intercalation of halloysite from Djebel Debagh (Algeria) and adsorption of copper ions. Applied Clay Science 44, 230 (2009).
- Phothitontimongkol T, Siebers N, Sukpirom N, Unob F. Preparation and characterization of novel organo-clay minerals for Hg(II) ions adsorption from aqueous solution Applied Clay Science 43, 343 (2009).
- Li S.Z, Wu P.X. Characterization of sodium dodecyl sulfate modified iron pillared montmorillonite and its application for the removal of aqueous Cu(II) and Co(II). Journal of Hazardous Materials 173, 62 (2010).
- Reddy M.L.P, Varna R.L, Ramamohan T.R, Damodaran A.D, Thakur P, Chakravortty V, Dash K.C. Synergistic solvent extraction of trivalent lanthanides with mixtures of 3 – phenyl – 4 – benzoyl – 5 – isoxazalone and crown ethers Solv. Extr. Ion Exch. 15 49 (1997)
- Manchada. V, Mohapatra. P.K, Veeraraghavan. R. 3-Phenyl-4-benzoyl-5-isoxazolone: A promising chelate extractant for actinide separation from acidic nuclear waste solutions. Anal. Chim. Acta, 320, 151 (1996)
- Messaoudi A, Torkestani K, Goetz-Grandmont. G, Brunette. J.P. J. Synergic extraction of alkaline earth cations with 3-phenyl-4-benzoyl-isoxazol-5-one and tri-n-octylphosphine oxide in toluene. Radioanal. Nucl. Chem 208 123 (1996)
- Boos. A, Intasiri. A, Brunette. J.P, Leroy. M.J.F. Surfactant-templated silica doped with 1-phenyl-3-methyl-4-stearoylpyrazol-5-one (HPMSP) as a new extractant. J. Mater. Chem. 12, 886 (2002)
- Todorova. O, Ivanova. E, Terebenina. A, Iordanov. N, Dimitrova. K, Borisov. G New chelating sorbents based on pyrazolone containing amines immobilized on styrene-divinylbenzene copolymer. I. Synthesis and analytical characterization Talanta 36, 817 (1989)
- Tong. A, Akama. Y, Tanaka. S. Preconcentration of indium with 1- phenyl-3-methyl-4-stearoyl-5-pyrazolone on silica gel.Anal. Chim. Acta 230, 175 (1990)
- Tong. A, Akama. Y, Tanaka. S. Preconcentration of copper, cobalt and nickel with 3-methyl-1-phenyl-4-stearoyl-5-pyrazolone loaded on silicagel. Analyst (Cambridge, UK) 115, 947 (1990)
- Tong. A, Akama. Y Preconcentration of trace metals with 1-phenyl-3- methyl-4-stearoyl-5-pyrazolone loaded on silica gel.Anal. Sci. 7, 83 (1991)
- Gao. J, Pan. Z, Du. X, Kang. J, Bai. G.Solid–liquid extraction of thorium and scandium complexes with 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone and its analytical application, Fenxi Huaxue 20 297 (1992)BY NATURAL BENTONITE
- Ivanova. E, Todorova. O, Stoimenova M New chelating sorbents of the pyrazolone type based on styrene-divinylbenzene copolymer. II. Sorptionof macroamounts of gold, palladium and silver.J. Anal. Chem. 344 316 (1992).
- Bou-Maroun E, Goetz-Grandmont G.J, Boos A. Sorption of europium(III) and copper(II) by a mesostructured silica doped with acyl-hydroxypyrazole derivatives: Extraction, kinetic and capacity studies. Colloids and Surfaces A: Physicochemical and Engineering Aspects 287, 1 (2006)
- Miloudi H, Boos A, Bouazza D, Ali-Dahmane T, Tayeb A, Goetz-Grandmont G, Bengueddach A. Acylisoxazolone-impregnated Si-MCM-41 mesoporous materials as promising liquid–solid extractants of metals. Materials Research Bulletin 42 769 (2007)
- Zhengshui H, Ying P, Wanwu M, Xun F. Purification of organophosphorus acid extractants. Solvent Extraction and Ion Exchange 13, 965 (1995).
- Tayeb A, Goetz-Grandmont G.J, Brunette J.P, Leroy M.J.F. Analytical and spectroscopic study of zinc extraction with 1,10-bis[1-phenyl-3-methyl-5-hydhoxy-1-pyrazolyl]-1,10-decanedione and tri-n-octylphosphine oxide. Solvent Extraction and Ion Exchange 8, 1 (1990).
- Jensen B.S. Acta Chem. Scand., 13, 1668 (1959).
- Chegrouche S, Mellah A, Telmoune S. Removal of lanthanum from aqueous solutions by natural bentonite Water Res 31, 1733 (1997).
- Sole K.C, Hiskey J.B. Solvent extraction of copper by Cyanex 272, Cyanex 302 and Cyanex 301. Hydrometallurgy 37, 129 (1995).
- Chah S, Kim J.S, Yi J.H. Separation of zinc ions from aqueous solutions using modified silica impregnated with Cyanex 272. Separation Science and Technology 37, 701 2002.
- Shiau C.Y, Lin C.L, Chang H.S. Adsorption Equilibrium of Zinc from Aqueous Sulfate Solution by Solvent-Impregnated Resins Containing Cyanex 272. Industrial & Engineering Chemistry Research 44, 4771 (2005).
- Northcott K, Kokusen H, Komatsu Y, Stevens G. Synthesis and surface modification of mesoporous silicate SBA-15 for the adsorption of metal ions. Separation Science and Technology 41, 1829 (2006).
- Fazlul Bari, Noorzahan Begum, Shamsul Baharin Jamaludin, Kamarudin Hussin. Synthesis of sol-gel silica chemically bonded with cyanex 272 for the removal of Cu(II), Ni(II), and Zn(II). Journal materials sciences 44, 2628 (2009).
- Northcott K, Syunichi Oshima, Jilska Perera, Yu Komatsu and Geoff Stevens. Synthesis, characterization and evaluation of mesoporous silicates for adsorption of metal ions. advandced powder technology 18, 751 (2007).
- Mansur M.B, Rocha S.D.F, Magalhaes F.S, Benedetto J.d.S. Selective extraction of zinc(II) over iron(II) from spent hydrochloric acid pickling effluents by liquid-liquid extraction. Journal of Hazardous Materials 150, 669 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









