Comparative Studies of Cerium and Thorium Soap Solution
Ramakant Sharma
Department of Chemistry, P.G. College, Ambah, Morena 476111, India.
DOI : http://dx.doi.org/10.13005/ojc/290344
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
The Ultrasonic velocity of cerium and thorium soaps has been measured in benzene-methanol mixture (50-50%) at a constant temperature (40 0.050C)/ The ultrasonic velocity and density data have been used to evaluate the adiabatic compressibility, intermolecular free length, molar sound velocity, specific acoustic impedance and other acoustic parameters cerium and Thorium soap solutions obey Bachem’s relation. The result Confirm that cerium and thorium soaps act as weak electrolyte in dilute solution below the CMC. The values of CMC are in agreement with the values obtained from other properties.
KEYWORDS:Cerium Thorium Laurate;Myrestate
Download this article as:| Copy the following to cite this article: Sharma R. Comprative Study Infrared of Cerium and Thorium Soaps. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290344 |
| Copy the following to cite this URL: Sharma R. Comprative Study Infrared of Cerium and Thorium Soaps. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=350 |
INTRODUCTION
Metal soaps are used as stabilizers for nylon threads, PVC molding composition, polyester, as an adhesive for steel cord and rubber in radial tiers1-4 The structure and properties 5-7of these soaps play And important role of their applications in different fields .Ultrasonic velocity have been found wide applications in owing to their ability to characterise the physico–chemical behaviour of solution .The velocity of ultrasonic waves in aqueous and non aqueous solution of electrolytes have been reported by several workers8-10.
In the present Communication attempts have been made to compute various acoustic parameters from ultrasonic velocity measurements of cerium thorium soap (laurate myrestate) Solutions .
EXPERIMENTAL
All the chemicals used for the preparation of cerium and thorium soaps were of BDH/AR grade and were purified by standard methods. Cerium and Thorium soaps was prepared by direct metathesis of the corresponding soap with the required amount of aqueous solution of cerium nitrate and thorium nitrate with constant stirring. The precipitated soap was filtered off and washes first with distilled water and finally with alcohol. The soaps were first dried in an air-oven at 50-600C and then under reduced pressure and further purified by recrystallisation. The solution of cerium and thorium soaps were preapared by dissolving the weighed amount of soaps in the required volume of benzene methanol mixture (50-50%). The solutions were kept at a constant temperature for two hours in a thermostat. The ultrasonic velocity measurements were recorded on an ultrasonic interferometer (M-83, Mittal Enterprises, New Delhi) at a frequency of 1 MHZ at a constant temperature (40 0.050C).
The probable error in velocity result was 0.2%.The various acoustic parameters such as adiabatic compressibility. β, intermoluecular free length, L, specific acoustic impedance, Z and molar sound viocity, R have been calculated by using the following relationships.
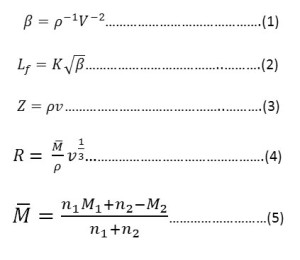
Where n1,n2 and M1, M2 are the number of moles and molecular weights of solvent and solutions respectively. K is Jacobson’s constant11
RESULT AND DISCUSSION
The ultrasonic velocity, v of Cerium and thorium soap solutions increases with the increasing concentration of soap in solution (Tables 1-4). The variations of velocity, v with concentration, C depends on concentration derivatives of ρ and β
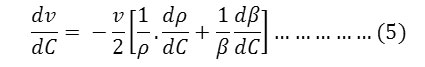
The results show that the density increases while the adiabatic compressibility decreases with increasing soap concentration. Therefore, the quantity ( dρ / dC )is positive while ( dβ / dC) is negative. Since the values of 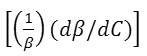 are larger then
are larger then ![]() for cerium and thorium soap solutions, thus the concentration derivative of velocity, dv/dC is positive, i.e. the ultrasonic velocity increases with increasing soap concentration.
for cerium and thorium soap solutions, thus the concentration derivative of velocity, dv/dC is positive, i.e. the ultrasonic velocity increases with increasing soap concentration.
The plots of ultrasonic velocity, v Vs soap concentration, C (Fig :1) are characterized by an intersection of two straight lines corresponding to the CMC of the soap. The values of the CMC are 6.45X10-4,6.20X10-4,, 6.10X10-4 and 6.00X10-4 (gmol dm-3 )for Cerium laurate cerium myrestate and thorium laurate, thorium myrestate, respectively. The plot are extrapolated value of velocity, V0(1.102X10-5 cm/Scc) in agreement with the experimental value of velocity of the solvent which indicated the aggregations of the soap molecules to an appreciable extent does not take place below the CMC.
The adiabatic compressibility, β of cerium and thorium soap solutions in benzene-methanol (50-50%) mixture decreases with increases in the soap concentration and increases in the chain length of the soap. The soaps behave as weak electrolytes in solution and iozine into simple metal cations, Ce3+ ,Th4+, and fatty acid anion, RCOO where R is C11H23, C13H27 The decrease in adiabatic compressibility may be attributed to the fact that the ions in solution are surrounded by a layer of solvent molecules firmly bound and oriented toward the ions. The orientation of the solvent molecules around the ionsn due to the influence of the electrostatic field of the ions, thus the internal pressure increases, which lowers the compressibility of solution i.e. , the solution becomes more difficult to compress.
The plots of adiabatic compressibility, β Vs soap concentration. C and the specific acoustic impedance. Z vs concentration, C are also characterized by an intersection of two straight lines which corresponds to the CMC of the soap. These plots have been extrapolated to zero soap concentration and the extrapolated value of adiabatic compressibility and specific acoustic impedance are in close agreement with the calculated values for the solvent
The results of the adiabatic compressibility have been explained in terms of Bachems’s12 equation
β = βO + AC – BC3/2
Where A and B are the constants, C is the molar concentration of the soap and β = βO are the adiabatic compressibility of the solution and solvent, respectively. The values of constants A and B have been obtained from the intercept and slope of the plots ( β – βO) CVs C1/2. The values of A are -14.52X10-9, -11.72X10-9, -10.44X10-9 and 9.20X10-9 for, Cerium laurate cerium myrestate and thorium laurate, thorium myrestate, respectively while the values of B are 133X10-9, -122X10-9, -100X10-9 and -80X10-9 for Cerium laurate cerium myrestate and thorium laurate, thorium myrestate respectively.
The intermolecular free length Lf for cerium and thorium soaps decreases with increasing soap concentration, which may be due to the decrease in adiabatic compressibility of the soap solution with increase in the soap concentration. The plots of Lf Vs soap concentration are characterized by the CMC of the soaps. The extrapolated values of Lf are in agreement with the calculated values Lf for benzene-methanol mixture. The values of intermolecular free length. Lf decrease with increasing ultrasonic velocity according to Erying and Kincoid13, which indicates a significant interaction between solute and solvent molecules, which affects the structural arrangement of the soap molecules.
The variation in the values of molar sound velocity, R with soap concentration are recorded (Table 1-4) The molar sound velocity decreases up to the CMC and then increases above the CMC.
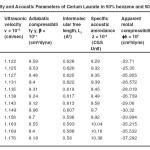 |
Table – 1 : Ultrasonic Velocity and Acoustic Parameters of Cerium Laurate in 50% benzene and 50% methanol at 40 + 0.05ºC. Click here to View table |
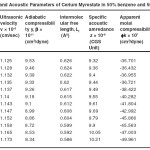 |
Table – 2 : Ultrasonic Velocity and Acoustic Parameters of Cerium Myrestate in 50% benzene and 50% methanol at 40 + 0.05ºC. Click here to View table |
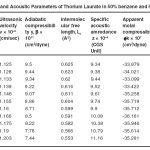 |
Table – 3 : Ultrasonic Velocity and Acoustic Parameters of Thorium Laurate in 50% benzene and 50% methanol at 40 + 0.05ºC. Click here to View table |
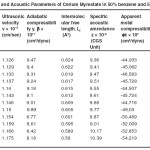 |
Table – 4 : Ultrasonic Velocity and Acoustic Parameters of Cerium Myrestate in 50% benzene and 50% methanol at 40 + 0.05ºC. Click here to View table |
 |
Figure 1 : Ultrasonic Velocity Vs. Concentration Click here to View figure |
REFERENCES
- Takekatsu, K., Hideka, S., Kazuo, M.l., Jpn Kokai Tokkyo koho., Jp 11.279-579 (1999), 11:302, 682(1999)
- Yutaka, N., and Yoshiyuki, T., Jpn. Kokai Tokkyo koho., JP: 294, 998 (1998).
- Saori, S., Mishika, I., and Kohol, S., Jpn. Kokai Tokkyo Koho., Jp 10:247, 828(2000).
- Lee, W.M., Albert Lee, H.W., Aworg Lee. H.Y. and Zhon N. Revista Lee Education Lee Lag Cien Cian., 3:37(2002)
- Sharma, V., Sharma . M and Sagar, V., J. Indian Chem. Soc. 83:1270(2006)
- Sagar,V., Sharma, M. And Sharma,V., Asian J.Chemistry, 18(4) : 3065 (2006)
- Mehta, V.P., Taleshara P.R., and Sharma R., Indian J.Chem. Sect. Lnorg, Bio- Inorg, Phys. Theor, Anal. Chem., 40A(4): 399 (2001)
- Prakash, O. And Prakash, S., J. Acoust . Soc. India, 6: 1139(1978).
- Mehrotra, K.N., Gahlaut A.S. , and Sharma M., J. Colloid interface Sci., 120:111(1987)
- Prakash, S., Prasad, N., Prakash, Orient. J. Chem. Eng. Data. 22:51(1977)
- Jacobson, B., Acta Chem. Scand., 6:14857 (1952)
- Bachem, C., Z. Phys.,101:541(1936).
- Erying H. and Kincold, J. Chem. Phys 6:620 (1938)
- Prakash, S. Singh, S., Prasad, N. And Singh R., Rev. Acust. 9:34 (1978)

This work is licensed under a Creative Commons Attribution 4.0 International License.









