An Efficient and Green Synthesis af Novel Azo Schiff Base and its Complex Under Ultrasound Irradiation
Mohammad Nikpassand*1, Leila Zare Fekri2 And Shohreh Sharafi1
1Department of Chemistry, Rasht Branch, Islamic Azad University, Rasht, Iran 2Department of Chemistry, Payame Noor University, PO Box 19395-3697 Tehran, Iran
DOI : http://dx.doi.org/10.13005/ojc/290326
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
An environmentally benign protocol for the synthesis of azo Schiff bases and its complex in Short reaction time and high yield has been achieved. These Schiff base compounds have been characterized by C, H, N elemental analysis, FT-IR and 1H NMR spectroscopy. The synthesized complex was characterized by atomic absorption and elemental analysis.
KEYWORDS:azo Schiff base;green synthesis;ultrasound irradiation
Download this article as:| Copy the following to cite this article: Nikpassand M, Fekri L. Z, Sharafi S. An Efficient and Green Synthesis af Novel Azo Schiff Base and its Complex Under Ultrasound Irradiation. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290326 |
| Copy the following to cite this URL: Nikpassand M, Fekri L. Z, Sharafi S. An Efficient and Green Synthesis af Novel Azo Schiff Base and its Complex Under Ultrasound Irradiation. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=311 |
INTRODUCTION
Schiff bases are well-known to have biological activates such as antibacterial1-2, antifungal3-4, antitumor5-6, antiviral7-8, anti bacterial7, antifungal7, anti-HIV-19, herbicidal10 and anti-influenza A virus11 activities.
Perhaps the most common method for preparing Schiff bases is the reaction of aldehydes and ketones with primary amines12. The reaction is generally carried out by refluxing the carbonyl compounds and amines in organic solvents. Recent years have witnessed a major drive to increase the efficiency of organic transformations while lowering the amount of waste materials Furthermore; the ultrasound assisted reactions are green methods in the organic synthesis which have numerous advantages: reduced pollution, low costs, and simplicity in process and handling13-14.
EXPERIMENTAL
Materials and instruments
For the ultrasound reactions, ultrasound apparatus astra 3D (9.5 dm3, 45 kHz frequency, input power with heating, 305W, number of transducers, 2) from TECNO-GAZ was used. Chemicals were purchased from Merck and Fluka and used as purchased. Melting points were measured on an Electro-thermal 9100 apparatus and are uncorrected. 1H NMR spectra were obtained on a Bruker DRX 500Avance spectrometer in DMSO-d6 as solvent and with TMS as internal standard. FT-IR spectra were recorded on a Shimadzu FT-IR-8400S spectrometer. Elemental analyses were recorded on a Carlo-Erba EA1110CNNO-S analyzer.
General Procedure for the synthesis of azo Schiff base
A mixture of azo-linked aldehydes (1mmol), aminopyrazole (1mmol) and 10 mL EtOH were placed into Pyrex-glass open vessel and irradiated in a water bath under silent condition by ultrasound (45 kHz) at 60°C for the required reaction times (5-15min). The progress of the reaction was monitored by TLC (EtOAc: petroleum ether 1:4). Then the reaction mixture was filtered to separate the product and recrystallized from EtOH. The pure products were collected in 85-96% yields.
4-((4-chlorophenyl)diazenyl)-2-((E)-(3-methyl-1H-pyrazol-5-ylimino)methyl) phenol 1: yellow solid, Mp 230 °C, IR (KBr): 3274, 2921, 1614, 1573, 1483, 1346, 1278, 1151 cm-1. 1HNMR (400 MHz, DMSO-d6, ppm): δH 2.28 (3H, s), 6.33 (1H, s), 7.15 (1H, J = 8.8 Hz, d), 7.66 (2H, J = 8.6 Hz, d), 7.83 (2H, J = 8.6 Hz, d), 7.99 (1H, J = 2 Hz, J = 8.8 Hz, dd), 8.28 (1H, J = 2.4 Hz, d), 9.21 (1H, s), 12.66 (1H, s), 13.95 (1H, s). 13C NMR (100 MHz, DMSO-d6, ppm): δC 25.60, 95.36, 118.39, 119.93, 124.41, 127.38, 128.06, 130.05, 135.81, 140.71, 145.21, 151.03, 161.53. Anal. Calcd. for C17H14ClN5O: C, 60.09; H, 4.15; N, 20.61. Found: C, 60.43; H, 4.11; N, 20.09.
2-((E)-(3-methyl-1H-pyrazol-5-ylimino)methyl)-4-((4-nitrophenyl)diazenyl)phenol 2: IR (KBr): 3407, 3213, 3132, 1614, 1434, 1517, 1361, 1286 cm-1. 1HNMR (400 MHz, DMSO-d6, ppm): δH 2.07 (3H, s), 6.30 (1H, s), 7.12 (1H, J = 8.7 Hz, d), 8.01 (3H, J = 8.2 Hz, d), 8.39 (2H, J = 8.2 Hz, d), 8.30 (1H, J = 3 Hz, d), 9.18 (1H, s), 12.64 (1H, s), 14.16 (1H, s). 13C NMR (100 MHz, DMSO-d6, ppm): δC 23.46, 93.43, 119.10, 124, 125.90, 128.08, 128.93, 129.46, 143.51, 144.74, 145.71, 148.85, 156.16, 161.31. Anal. Calcd. for C17H14N6O3: C, 58.28; H, 4.03; N, 23.99. Found: 58.65; H, 4.25; N, 23.09.
2.2.3. 2-((E)-(3-methyl-1H-pyrazol-5-ylimino)methyl)-4-(phenyldiazenyl)phenol 3:IR (KBr): 3438, 3089, 2974, 1614, 1560, 1490, 1436, 1282 cm-1. 1HNMR (400 MHz, DMSO-d6, ppm): δH 2.05 (3H, s), 6.33 (1H, s), 7.15 (1H, J = 8.8 Hz, d), 7.55-7.62 (3H, m), 7.87 (2H, J = 7.2 Hz, m), 7.99 (1H, J = 2 Hz, J = 8.8 Hz, dd), 8.28 (1H, s), 9.21 (1H, s), 12.65 (1H, s). 13C NMR (100 MHz, DMSO-d6, ppm): δC 25.6, 96.30, 107.15, 116.12, 118.91, 122.69, 123.32, 123.71, 124.91, 129.92, 138.43, 139.66, 155.41, 161.30. Anal. Calcd. for C17H15N5O: C, 66.87; H, 4.95; N, 22.94. Found: 66.35; H, 4.75; N, 22.42.
4-((4-bromophenyl)diazenyl)-2-((E)-(3-methyl-1H-pyrazol-5-ylimino)methyl) phenol 4: IR (KBr): 3448, 3274, 1614, 1558, 1278, 1151, 1002 cm-1. 1HNMR (400 MHz, DMSO-d6, ppm): δH 2.28 (3H, s), 6.35 (1H, s), 7.15 (1H, J= 8.8 Hz, d), 7.80 (4H, br), 7.99 (1H, J= 8.8 Hz, d), 8.28 (1H, s), 9.20 (1H, s), 12.66 (1H, s). 13C NMR (100 MHz, DMSO-d6, ppm): δC 23.83, 95.35, 118.44, 119.91, 122.36, 124.64, 127.39, 128.91, 132.99, 140.43, 145.20, 151.33, 161.24. Anal. Calcd. for C17H14BrN5O: C, 53.14; H, 3.67; N, 18.23. Found: C, 53.37; H, 3.73; N, 18.96.
4-((2-chlorophenyl)diazenyl)-2-((E)-(3-methyl-1H-pyrazol-5-ylimino)methyl) phenol 5: IR (KBr): 3429, 3128, 2928, 1616, 1502, 1456, 1325, 1280, 1054 cm-1. 1HNMR (400 MHz, DMSO-d6, ppm): δH 2.28 (3H, s), 6.35 (1H, s), 7.17 (1H, J = 8.8 Hz, d), 7.48-7.57 (2H, m), 7.67-7.72 (2H, m), 8.01 (1H, J = 2 Hz, J = 8.8 Hz, dd), 8.31 (1H, J = 2.4 Hz, d), 9.23 (1H, s), 12.66 (1H, s), 14.08 (1H, s). 13C NMR (100 MHz, DMSO-d6, ppm): δC 95.41, 118.04, 118.64, 119.87, 127.32, 128.56, 128.97, 131.17, 132.51, 133.86, 140.76, 145.54, 148.46, 156.16, 161.62, 164.64. Anal. Calcd. for C17H14ClN5O: C, 60.09; H, 4.15; N, 20.61. Found: C, 60.56; H, 4.01; N, 20.45.
4-((4-iodophenyl)diazenyl)-2-((E)-(3-methyl-1H-pyrazol-5-ylimino)methyl)phenol 6: IR (KBr): 3419, 1610, 1560, 1469, 1276, 1101 cm-1. 1HNMR (400 MHz, DMSO-d6, ppm): δH 2.28 (3H, s), 6.33 (1H, s), 7.15 (1H, J = 9 Hz, d), 7.66 (2H, J = 8.6 Hz, d), 7.99 (2H, J = 8.6 Hz, d), 8.01 (1H, J = 8.8 Hz, J = 2 Hz, dd) ,8.28 (1H, J = 2.4 Hz, d), 9.21 (1H, s), 12.66 (1H, s), 14.00 (1H, s). Anal. Calcd. for C17H14IN5O: C, 47.35; H, 3.27; N, 16.24. Found: C, 47.76; H, 3.11; N, 16.61.
2-((E)-(3-methyl-1H-pyrazol-5-ylimino)methyl)-4-(p-tolyldiazenyl)phenol 7: IR (KBr): 3502, 3163, 3082, 2968, 1606, 1568, 1446, 1278. 1HNMR (400 MHz, DMSO-d6, ppm): δH 2.28 (3H, s), 2.41 (3H, s), 6.33 (1H, s) 7.14 (1H, J = 8.8 Hz, d), 7.40 (2H, J = 8.4 Hz, d), 7.77 (2H, J = 8.4 Hz, d), 7.96 (1H, J = 2 Hz, J = 8.8 Hz, d), 8.24 (1H, J = 2 Hz, d), 9.21 (1H, s), 12.65 (1H, s), 13.85 (1H, s). 13C NMR (100 MHz, DMSO-d6, ppm): δC 21.48, 25.60, 95.32, 118.25, 119.86, 122.56, 122.77, 127.21, 127.61, 130.43, 141.58, 145.35, 150.51, 161.75. Anal. Calcd. for C18H17N5O: C, 67.70; H, 5.37; N, 21.93;. Found: C, 67.30; H, 5.83; N, 21.11.
4-((3-chlorophenyl)diazenyl)-2-((E)-(3-methyl-1H-pyrazol-5-ylimino)methyl) phenol 8: IR (KBr): 3274, 3448, 2925, 1652, 1558, 1458, 1217, 756 cm-1. 1HNMR (400 MHz, DMSO-d6, ppm): δH 2.09 (3H, s), 6.33 (1H, s), 7.67-7.71 (2H, m), 7.96 (2H, m), 8.08 (1H, J = 9.2 Hz, d), 8.28 (1H, J = 9.2 Hz, d), 8.89 (1H, s), 9.21 (1H, s), 13.41 (1H, s). 13C NMR (100 MHz, DMSO-d6, ppm): δC 25.60, 95.43, 120.66, 121.37, 122.97, 123.62, 129.84, 131.33, 131.82, 132.66, 132.79, 135.01, 138.36, 147.46, 149.72, 152.08, 161.34. Anal. Calcd. for C17H14ClN5O: C, 60.09; H, 4.15; N, 20.61;. Found: C, 60.39; H, 4.16; N, 20.81.
2-((E)-(3-methyl-1H-pyrazol-5-ylimino)methyl)-4-((2-methyl-4-nitrophenyl) diazenyl)phenol 9: IR (KBr): 3274, 3149, 3191, 2991, 1614, 1519, 1434, 1575, 1342, 1288, 1115 cm-1. 1HNMR (400 MHz, DMSO-d6, ppm): δH 2.25 (3H, s), 2.69 (3H, s), 6.26 (1H, s), 7.08 (1H, br), 7.60 (1H, br), 7.92 (1H, br), 8.08 (1H, br), 8.21 (2H, br), 9.10 (1H, s), 12.62 (1H, s), 14.13 (1H, s). 13C NMR (100 MHz, DMSO-d6, ppm): δC 17.89, 23.50, 95.81, 117.29, 118.97, 120.12, 127.04, 127.65, 129.92, 130.01, 139.10, 141.07, 146.05, 148.51, 154.21, 155.12, 157.46, 161.72. Anal. Calcd. for C18H16N6O3: C, 59.34; H, 4.43; N, 23.07. Found: C, 59.35; H, 4.82; N, 23.49.
4-((4-methoxyphenyl)diazenyl)-2-((E)-(3-methyl-1H-pyrazol-5-ylimino)methyl) phenol 10: IR (KBr): 3274, 3448, 1652, 1558, 1458, 1382, 1217 cm-1. 1HNMR (400 MHz, DMSO-d6, ppm): δH 2.40 (3H, s), 3.74 (3H, s), 6.20 (1H, s), 7.16 (1H, J = 8.8 Hz, d), 7.53 (2H, J = 8.4 Hz, d), 7.91 (2H, J = 8.4 Hz, d), 8.44 (1H, J = 8.4 Hz, d), 8.66 (1H, s), 9.48 (1H, s), 13.60 (1H, s) ppm. Anal. Calcd. for C18H17N5O2: C, 64.47; H, 5.11; N, 20.88. Found: C, 64.49; H, 5.61; N, 20.73.
Preparation of complex 4i under ultrasound irradiation
A mixture of Schiff base 3i (2mmol), Cu(OAc)2 (1mmol) and 10 mL EtOH were placed into Pyrex-glass open vessel and irradiated in a water bath under silent condition by ultrasound (45kHz) at 60°C for the required reaction times (15min). The progress of the reaction was monitored by TLC (EtOAc: petroleum ether 1:4). Then the reaction mixture was filtered to separate the product and recrystallized from EtOH. The pure products were collected in 98% yields. Anal. Calcd. for C36H32Ac2CuN12O82-: C, 33.46; H, 2.56; N, 13.42. Found: C, 33.83; H, 2.52; N, 13.15.
RESULTS AND DISCUSSION
As part of our going interest for the development of efficient and environmentally friendly procedures for the synthesis of heterocyclic and pharmaceutical compounds15-18 we wish to report the first ultrasound assisted synthesis of novel derivatives of Schiff bases (Scheme 1).
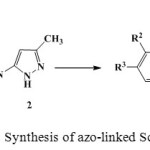 |
Scheme 1: Synthesis of azo-linked Schiff bases Click here to View figure |
In an initial endeavor, 1a and 5-methyl-3-aminopyrazole were refluxed in 10 mL ETOH. After 7 h only, 73 % of expected product after purification of crude product was obtained. To improve the product yields and to optimize the reaction condition, the same reaction was carried out in the presence of ultrasonic’s irradiation. We found that the best results were obtained under ultrasound irradiation at 60 °C in EtOH. As indicated in Table 1, ultrasonic irradiation accelerates the reaction, the reaction time decreases from 7 h to 8min (Entry 1, Table 1). Also, under ultrasonic irradiation the yield of product is higher. The results are summarized in table 1. The higher yield and less reaction time during ultrasonic irradiation in the presence of the catalyst can be attributed to implosive collapse of the cavitations period of the sound waves. When the formed bubbles burst, it results in high temperature and high pressure which facilitate the intermolecular reaction.
In the other study, we concentrated on the synthesis of novel complex of this Schiff base in the reflux condition and ultrasound irradiation. The structure of synthesized complex 4i was shown in Scheme 2.
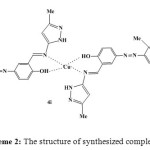 |
Scheme 2: The structure of synthesized complex 4i Click here to View figure |
All of compounds summarized in table 1 were characterized by spectroscopic methods (IR,1H NMR, 13C NMR) and elemental analysis. In the 1H NMR spectra of azo-linked Schiff bases 3a-j, imines C-H proton of resonated at 6.2-6.3 ppm and in the 13C NMR spectra of azo-linked Schiff bases 3a-j, imines C-H carbon of resonated at 95 ppm.
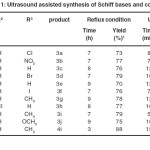 |
Table 1. Ultrasound assisted synthesis of Schiff bases and complex Click here to View table |
Following these results, we further investigated the potential of this procedure for the selective synthesis of azo-linked Schiff bases of different types of azo-linked aldehydes with pyrazolamines. The results showed that ultrasound irradiation is able to discriminate between aromatic compounds containing electron donating and electron withdrawing groups, a transformation that is difficult to accomplish via conventional methods (Scheme 3).
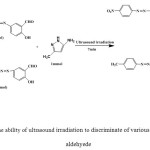 |
Scheme 3: The ability of ultrasound irradiation to discriminate of various substituted azo aldehyede Click here to View figure |
CONCLUSION
Finally, we develop an efficient, green, fast and convenient procedure for the synthesis of azo-linked Schiff bases through electrophilic substitution of azo-linked aldehydes and pyrazole amine under ultrasound irradiation. This procedures offer advantages such as reduced reaction time, mild reaction condition, productivity and higher yield, ease of execution and economic viability. To the best of our knowledge, the process described herein represents the first example of ultrasound assisted synthesis of azo-linked Schiff bases and its complex.
ACKNOWLEDGEMENTS
We thank the Research Committee of Islamic Azad university of Rasht branch for partial support given to this study.
REFERENCES
- Panneerselvam P., Rather B. A., Reddy D. R. S. and Kumar, N. R., Eur. J. Med. Chem., 44: 2328 (2009).
- Singh K.; Barwa M. S. and Tyagi P., Eur. J. Med. Chem., 44: 147 (2006).
- Panneerselvam P., Nair R. R., Vijayalakshmi G. E., Subramanian H. and Sridhar S. K., Eur. J. Med. Chem., 40: 225 (2005).
- Sridhar S. K., Saravan M. and Ramesh A., Eur. J. Med. Chem., 36: 615 (2001).
- Walsh O. M., Meegan M. J., Prendergast R. M. and Nakib T. A., Eur. J. Med. Chem., 31: 989 (1996).
- Hodnett E. M. and Dunn W. J., J. Med. Chem., 13: 768 (1970).
- Kumar K. S., Ganguly S. and Veerasamy R., Eur. J. Med. Chem., 45: 5474 (2010).
- Jarrahpour A., Khalili, D., De Clercq E., Salmi C. and Brunel J. M., Molecules, 12: 1720 (2007).
- Vicini P., Geronikaki A., Incerti M., Busonera B., Poni G., Cabras C. A. and La Colla P., Bioorg. Med. Chem., 11: 4785 (2003).
- Holla B. S., Rao B. S., Shridhara K., and Akberali P. M., IL Farmaco, 55: 338 (2000).
- Zhao X., Li, C., Zeng S. and Hu W., Eur. J. Med. Chem., 46: 52 (2011).
- Tidwell T. T., Angew. Chem. Int. Ed., 47: 1016 (2008).
- Tanaka K. and Toda F., Chem. Rev., 100: 1025 (2000)..
- Schmeyers J., Toda F., Boy J. and Kaupp G., J. Chem. Soc., Perkin Trans, 2: 989 (1998).
- Zare L. and Nikpassand M., Chin. Chem. Lett., 22: 531 (2011).
- Nikpassand M., Zare L. and Saberi M., Monatsh. Chem., 143: 289 (2012).
- Nikpassand M., Zare L., Shafaati T. and Shariati Sh., Chin. J. Chem., 30: 604 (2012).
- Nikpassand M., Zare L., Gharib M. and Marvi O., Lett. Org. Chem., 9: 745 (2012).

This work is licensed under a Creative Commons Attribution 4.0 International License.









