Thermodynamics of Micellization of Nonionic Surfactant Triton X-100 in Presence of Additive Poly-N-Vinyl- Pyrrolidone using Clouding Phenomenon
A. D. Mudawadkar1, G. H. Sonawane1* and T. J. Patil2
1*Department of Chemistry, KVP,S Kisan Arts Commerce and Science College, Parola, Dist. Jalgaon - 425 111, India. 2Department of Chemistry, Z.B. Patil College, Deopur, Dhule - 424 002, India.
The solvation and desolvation phenomenon of non-ionic surfactants, Iso-Octyl phenoxy polyethoxy-ethanol (Triton-X-100) have been studied by determining the cloud point (CP) at various surfactant concentrations in pure and mixed system with Poly-N-Vinyl 2 – Pyrrolidone (PVP). The CP of pure Triton X-100 was found to be increased with increasing surfactant concentration. The CP of pure surfactant (Triton-X-100) found to be decreased with increased concentration of additive PVP. The CP of mixed system also shows same trends with increased [PVP]. This is mainly due to increase in micelle concentration, also due to the removal of water molecules by added polymers which helps the surfactant micelles to come closer with each other resulting into lowering of CP of the present polymer surfactant systems. The phase separation results from micelle-micelle interaction. Considering CP as a threshold temperature of the solubility, the thermodynamic parameters of clouding process (#DELTA;G0cl, ΔH0cl and #DELTA;S0cl) have been evaluated using “Phase Separation Model” The findings of the present work supports to make the probable evidence of polymer-surfactant interactions in aqueous medium.
KEYWORDS:Micellization; Cloud Point (CP); Triton-X-100; Poly-N-Vinyl-2 Pyrrolidone (PVP); Phase separation model
Download this article as:| Copy the following to cite this article: Mudawadkar A. D, Sonawane G. H, Patil T. J. Thermodynamics of Micellization of Nonionic Surfactant Triton X-100 in Presence of Additive Poly-N-Vinyl- Pyrrolidone using Clouding Phenomenon. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Mudawadkar A. D, Sonawane G. H, Patil T. J. Thermodynamics of Micellization of Nonionic Surfactant Triton X-100 in Presence of Additive Poly-N-Vinyl- Pyrrolidone using Clouding Phenomenon. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25152 |
Introduction
The clouding of ionic/nonionic surfactants and polymers alone and mixture has been studied by several research workers.1-4 The physico chemical studies of polymer surfactant solution have created much interest regarding their industrial importance. 5-9 Non-ionic surfactants cannot withstand at elevated temperature and become perceptible even with the naked eye is known as “Clouding”. This temperature is referred as “Cloud Point” 10 (CP), the cloud point is as important is an important property of non-ionic surfactants, below CP a single phase of molecular solution or micellar solution exists; above CP the water solubility of surfactant is reduced and it results into cloudy dispersion.11 The CP values for a polymer-surfactant mixture may be a guide to its hydrophilic or hydrophobic character. When surfactants are added to water at low concentration, they are dispersed as discrete molecules. However at a particular concentration, surfactant molecules get associated to form aggregates or micelles12-14. This concentration is known as critical micellar concentration (CMC) which is an important property of surfactant. Above CMC the surfactant molecules exists as aggregates or micelles. CMC of surfactant is determined by several methods such as conductance, solubilization, surface tension etc.
In this paper the results of our study on the clouding phenomenon of pure Triton-X-100 and in presence PVP at various concentrations has reported. These studies are important in the field of medicinal preparations, agrochemicals, detergents, etc. considering cloud point as threshold temperature of the solubility, the thermodynamic parameters of clouding process (∆G0cl, ∆H0cl and ∆S0cl) have been evaluated using, “Phase Separation Model”
Materials and Methods
Nonionic surfactant Triton-X-100 was obtained from Fluka Chemie and it was used as received. Water soluble polymer (Poly-N -vinyl-2- Pyrrolidone) PVP was the product of Sigma, USA (Mol. Wt.25000 and 40000). Both the PVP polymers are dialyzed to remove low molecular weight fractions and other associated electrolytic impurities before use.
Doubly distilled water with specific conductance 2-4 x106 Scm-1 at 303.15 K was used in preparation of solutions of different concentrations.
The cloud point (CP) was determined by controlled heating in well stirred surfactant solution as well as surfactant-PVP mixture until it clouded or got turbid. The turbid solution was then allowed to cool slowly while being stirred and the temperature for the disappearance of turbidity was considered as the cloud point of the test solution. Heating and cooling was regulated to about 10C per minute around the CP. The reproducibility of the measurement was found to be within ± 0.20C. As the CP value are not small, the observed values have been rounded off to the nearest degree and presented in the tables.
Clouding species
Surfactant-
Iso-Octyl phenoxy polyethoxy – ethanol (Triton-X-100) (TX-100)
Polymers- (Additves)
Poly-N-Vinyl 2 – Pyrrolidone (PVP)
 |
Figure 1: Molecular structures of clouding species and additives. Click here to View figure |
![Figure 1a: Influence of [PVP] on CP of Troton-X-100 Mw-25000 from 1to 5 Wt %](http://www.orientjchem.org/wp-content/uploads/2013/03/Vol_29-no1_Ther_MUDA_fig1a-150x150.jpg) |
Figure 1a: Influence of [PVP] on CP of Troton-X-100 Mw-25000 from 1to 5 Wt % Click here to View figure |
![Figure 2: Influence of [PVP] on CP of Troton-X-100 Mw-25000 from 6 to 10Wt %](http://www.orientjchem.org/wp-content/uploads/2013/03/Vol_29-no1_Ther_MUDA_fig2-150x150.jpg) |
Figure 2: Influence of [PVP] on CP of Troton-X-100 Mw-25000 from 6 to 10Wt % Click here to View figure |
![Figure 3: Influence of [PVP] on CP of Troton-X-100 Mw-40,000 from 1to 5 Wt %](http://www.orientjchem.org/wp-content/uploads/2013/03/Vol_29-no1_Ther_MUDA_fig3-150x150.jpg) |
Figure 3: Influence of [PVP] on CP of Troton-X-100 Mw-40,000 from 1to 5 Wt % Click here to View figure |
![Figure 4: Influence of [PVP] on CP of Troton-X-100 Mw-40,000 from 6 to 10 Wt %](http://www.orientjchem.org/wp-content/uploads/2013/03/Vol_29-no1_Ther_MUDA_fig4-150x150.jpg) |
Figure 4: Influence of [PVP] on CP of Troton-X-100 Mw-40,000 from 6 to 10 Wt % Click here to View figure |
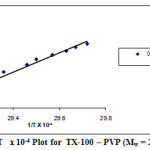 |
Figure 5: lnXs Vs 1/T x 10-4 Plot for TX-100 – PVP (Mw = 25000) for 0.005 % Click here to View figure |
Table 1: CP of Triton-X-100 (pure non-ionic surfactant) at different concentration (Wt %).
|
[Triton-X-100] Wt % |
Molarity x 102 |
Mole fraction x 104 |
ln Xs |
CP/0C |
|
1 |
1.548 |
2.783 |
-8.1867 |
63 |
|
2 |
3.096 |
5.565 |
-7.49384 |
64 |
|
3 |
4.644 |
8.338 |
-7.0895 |
64.5 |
|
4 |
6.192 |
11.124 |
-6-.80123 |
65 |
|
5 |
7.740 |
13.901 |
-6.5783 |
65.5 |
|
6 |
9.288 |
16.677 |
-6.39630 |
65.8 |
|
7 |
10.836 |
19.451 |
-6.2424 |
66 |
|
8 |
12.380 |
22.216 |
-6.10952 |
66.5 |
|
9 |
13.931 |
24.991 |
-5.99182 |
66.8 |
|
10 |
15.480 |
27.834 |
-5.8840 |
67.00 |
Result and Discussion
Cloud Points (CP) of Pure TX – 100:
The cloud points of TX-100 pure non ionic surfactant at different concentrations in (Wt %) are given in Table 1. The CP of TX-100 are substantially constant over a wide range of concentration. The values of CP increases mildly from 63oC to 67oC with increase in concentration of surfactant from 1 to 10 Wt%. In fact, CP of TX-100 has been reported to change very slowly15.
Cloud Points (CP) Triton X-100 – Poly (N-Vinyl-2 Pyrrolidone):
The influence of PVP (Mol. Wt. 25000) and PVP (Mol. wt. 40000) on the CP cloud point of TX-100 at different concentrations has been also studied. The results are given in Table 2 and Table 3. These results indicating that the cloud point of surfactant declined considerably with increased molecular weight of PVP from 25000 to 40000. It has been found that the low concentration of PVP, below 0.005 Wt % did not have much effect on the CP of pure Triton X-100 and it remains around 630C. With addition of PVP CP of T-X-100 increases Table 1 and 2. As [PVP] increases from 0.005 to 0.05 CP decreases from 69.6 to 66.80C. As concentration of T-X-100 increases from 1 to 10 Wt% CP decreases from 69.9 to 63.5oC for 0.005Wt% of PVP. As molecular weight of PVP was increased from 25,000 to 40,000 the CP of T-X-100 decreases with addition of PVP for all concentration pairs studied. The influence of [PVP] on CP of T-X-100 for Mw 25,000 and 40,000 are shown in a graph 1, 2 and 3 respectively.
The values of CP in Table 2 and Table 3 show that CP declines with increase PVP concentration effectively. This is mainly due to removal of water molecules by added polymers which helps the surfactant micelles to come closer with each other resulting into lowering of CP of the present [PVP] surfactant systems.
Table 2 : Influence of [PVP] on CP of Triton-X-100 Mw = 25000.
|
Triton X-100 Wt % |
CP0/C at [PVP] (Wt %) |
|||||
|
0.005 |
0.01 |
0.02 |
0.03 |
0.04 |
0.05 |
|
|
1 |
69.6 |
68.8 |
68.5 |
67.9 |
67.2 |
66.8 |
|
2 |
68.9 |
68.4 |
67.8 |
67.1 |
66.8 |
66.4 |
|
3 |
68.2 |
67.8 |
67.4 |
66.8 |
66.1 |
65.4 |
|
4 |
67.5 |
66.5 |
66.2 |
65.5 |
64.1 |
64.1 |
|
5 |
66.4 |
66.1 |
65.5 |
64.8 |
63.8 |
63.2 |
|
6 |
66.0 |
65.5 |
64.2 |
64.2 |
63.2 |
62.4 |
|
7 |
65.32 |
64.6 |
63.8 |
63.8 |
62.8 |
62.2 |
|
8 |
64.5 |
63.9 |
63.4 |
62.6 |
62.1 |
61.9 |
|
9 |
64.0 |
63.1 |
62.8 |
62.2 |
61.4 |
61.1 |
|
10 |
63.5 |
62.8 |
62.1 |
61.8 |
60.6 |
59.6 |
Table 2 : Influence of [PVP] on CP of Triton-X-100 Mw = 25000.
Triton X-100
Wt % |
CP0/C at [PVP] (Wt %) |
|||||
|
0.005 |
0.01 |
0.02 |
0.03 |
0.04 |
0.05 |
|
|
1 |
65.4 |
64.8 |
61.1 |
63.2 |
63.1 |
63.8 |
|
2 |
62.6 |
62.1 |
63.4 |
62.8 |
62.2 |
59.2 |
|
3 |
60.3 |
59.3 |
58.7 |
58.1 |
58.0 |
58.1 |
|
4 |
59.9 |
58.4 |
57.4 |
57.0 |
57.1 |
57.4 |
|
5 |
58.6 |
58.1 |
56.8 |
56.2 |
56.0 |
56.8 |
|
6 |
54.1 |
56.7 |
53.2 |
53.7 |
53.5 |
54.4 |
|
7 |
53.9 |
53.3 |
52.6 |
52.1 |
52.0 |
48.0 |
|
8 |
52.3 |
52.1 |
50.8 |
50.4 |
49.2 |
47.2 |
|
9 |
51.6 |
51.4 |
49.2 |
49.0 |
48.3 |
46.5 |
|
10 |
50.9 |
50.1 |
48.8 |
48.2 |
47.4 |
46.1 |
Thermodynamics of clouding
All physicochemical processes are energetically controlled. The spontaneous formation of micelle is obviously guided by thermodynamic principles. Cloud points are the characteristics of non-ionic surfactants. Thermodynamic parameters of pure Triton-X-100 are given in Table 4 and Triton-X-100 – PVP mixed systems are given in Table 5 and 6 respectively. In case of non-ionic surfactant the desolvation of hydrophilic groups of the surfactant leads to the formation of cloud turbidity in the surfactant solution at elevated temperature. The appearance of cloud point is entropy dominated. At the cloud point, the water molecules get detached from the micelles.
Table 4: Thermodynamic parameters of solubilization of TX-100.
| [Triton-X-100] Wt% DG0Cl
kJmole-1 |
–DH0Cl kJmole-1 |
–DS0Cl Jmole-1 K-1 |
| 1 22.86 |
689.55 |
|
| 2 20.99 |
681.94 |
|
| 3 19.89 |
677.67 |
|
| 4 19.11 |
674.36 |
|
| 5 18.51 |
208.82 |
671.59 |
| 6 18.01 |
669.53 |
|
| 7 17.59 |
667.89 |
|
| 8 17.24 |
665.88 |
|
| 9 16.92 |
664.35 |
|
| 10 16.63 |
663.09 |
Table 5: Thermodynamic Parameters of TX-100 in presence of PVP Mw =25,000.
|
Wt % [PVP] |
DG0Cl kJmole-1 |
–DH0Cl kJmole-1 |
–DS0Cl Jmole-1 K-1 |
|
0.05 |
48.82 |
113.82 |
474.70 |
|
0.01 |
46.71 |
109.38 |
456.71 |
|
0.02 |
44.71 |
107.07 |
444.47 |
|
0.03 |
43.47 |
108.44 |
446.69 |
|
0.04 |
42.42 |
106.86 |
438.80 |
|
0.05 |
41.81 |
101.44 |
421.57 |
Table 6: Thermodynamic parameters of TX-100 in presence of PVP Mw=40,000.
|
Wt % [PVP] |
DG0Cl kJmole-1 |
–DH0Cl kJmole-1 |
–DS0Cl Jmole-1 K-1 |
|
0.05 |
49.54 |
56.98 |
317.78 |
|
0.01 |
47.52 |
48.37 |
283.88 |
|
0.02 |
45.45 |
44.41 |
266.55 |
|
0.03 |
44.22 |
42.60 |
258.24 |
|
0.04 |
43.28 |
40.43 |
249.74 |
|
0.05 |
42.84 |
35.38 |
232.24 |
Considering cloud point as the phase separation point, the thermodynamic parameters such as standard free energy (∆G0cl), enthalpy (∆H0cl) and entropy (∆S0cl) for the clouding process have been calculated using the Phase Separation Model.16 The following relation can be written as –
∆G0cl = -RT ln Xs ……1
Where “cl” stands for clouding process and lnXs is the mole fractional solubility of the solute.
The standard enthalpy (∆H0cl) for the clouding process have been calculated from the slope of the linear plot of ln Xs Vs 1/T in Fig.1
d ln Xs/ dT = ∆H0cl / RT2 ……2
The standard free energy of the clouding process ∆S0cl have been calculated from the following relationship
∆S0cl = (∆H0cl – ∆G0cl )/T. ……3
The thermodynamic parameters for pure surfactant and in mixed systems are given in Table 4 and Table 5, 6.
∆H0cl < ∆G0cl indicating that overall clouding process is exothermic and also ∆H0cl > T∆S0cl indicate that the process of clouding is guided by both enthalpy and entropy 17.
The present work would be supportive evidence regarding the probable interaction between non-ionic surfactant and macromolecules, water soluble polymer leading to the phase separation at the cloud point. The effect of PVP on the cloud point is a clear indication that the phenomenon of clouding is associated with the different micelles coalescing.
Acknowledgement
The authors are thankful to Hon’ble Principal and Head, Department of Chemistry, Z.B. Patil College, Dhule. Also Hon’ble Principal and Head Department of Chemistry, Kisan Arts Commerce and Science College, Parola, Dist. Jalgaon for providing laboratory facilities.
References
- Maulik, S.P. and Soumen Ghosh, J. Mol. Lip., 72, 145 (1997).
- Robb, I. D. Anionic Surfactants – Physical Chemistry of Surfactant Action, Edited by E.H. Lucassen Rehynders Marcel Dekker, New York, (1981).
- Wang and Olofsson, G., J. Phys. Chem. 99, 5588 (1955).
- Molyneux, P. Water Soluble Synthetic Polymers: Properties and Behaviour, CRC Press, Boca Raton FL Vol. II Chapt. 2, (1984).
- Janes, M.N., J. Colloid Interface Sci. 23, 36 (1967)
- Goddard, E.D., Colloids Surf., 19, 255 (1986).
- Bloor, D.M. and Wyn. E. Jones, J. Chem. Soc. Faraday II, 78, 657, (1982).
- Chari, K., Antalek, B., Lin, M.Y. and Sinh S.K., J. Chem. Phys. 7, 100 (1994).
- Goddard, E.D., Interaction of Surfactants with Polymers and Proteins,Edited by Goddard, E.D. and Ananthpadmanabhan K.P., CRC Press, Baca Ratan, PL 123 (1993).
- Shinoda, K., Makagawa T., Tamamushi B. and Ishemushi T. Colloidal Surfactants, Academic Press, New York / Londan, 12 (1967).
- Arai, H., Murata, M. and Shinoda K., J. Colloidal Interface Sci. 37, 223 (1971).
- McBain J. W., Trans Farad Soc., 9, 99 (1913).
- Almgreh, M. and Swanup, S., J. Phy Chem. 86, 4212 (1982).
- Bellare, J.R., Kaneko, T. and Evans, D.F. Langmuir, 4, 1066 (1988).
- Shinoda, K., Makagawa, T. Tanamushi, B. and Semura, T. I., Colloidal Surfactant Some Physicochemical Properties, Academic Press, New York (1963).
- Attwood, D. and Flarance, At Surfactant Systems, Champman and Hall. London, 99 (1983).
- Patil T.J. and Patil H.A., Int. J. Chem. Sci. 3 (3), 507 (2005).

This work is licensed under a Creative Commons Attribution 4.0 International License.









