Synthesis of 1, 4-Dihydropyridine Derivatives using Fe [(L)proline]2 as Catalyst
Abbas Bidram*, Farhad Hatamjafari and Ali Doryeh
Department of Chemistry, Faculty of Science, Islamic Azad University-Tonekabon Branch, Tonekabon, Iran.
Article Received on :
Article Accepted on :
Article Published : 19 Oct 2016
A mixture of ethyl acetoacetate, benzaldehyde and ammonium acetate and in the presence of Fe [(L)proline]2 were converted to 1,4-dihydropyridines with good yields. IR spectra Confirms formation complex of Fe [(L)proline]2
KEYWORDS:1; 4-dihydropyridines; ammonium acetate; ethyl acetoacetate; Fe [(L) proline]2
Download this article as:| Copy the following to cite this article: Bidram A, Hatamjafari F, Doryeh A. Synthesis of 1, 4-Dihydropyridine Derivatives using Fe [(L)proline]2 as Catalyst. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Bidram A, Hatamjafari F, Doryeh A. Synthesis of 1, 4-Dihydropyridine Derivatives using Fe [(L)proline]2 as Catalyst. Available from: http://www.orientjchem.org/?p=22575 |
Introduction
Chemical dihdropyridins reported by arthur hantzsch from 1882 years, he compounded ᵝ-ketoester, aldehyde and ammohia that lead to forms of 1¡4-dihidropyridines where as reported many advantages of 1¡4-dihydropiridin ,and at this time identify as importance and vital in treatment of calcium antagonists1, antitumours2, antidiabetics3, antagonists4 and antivirals5. Recently a number of articles have published on the synthesis of 1, 4-dihydropyridines6-12. Heterogeneous catalysts have gained and have been widely used as a stable and an efficient catalyst for synthesis of organic compound.
We have synthesized of DHPs from ethyl acetoacetate, benzaldehyde, and ammonium acetate using Fe [(L)proline]2 as catalyst (Scheme 1). Once the reaction goes to completion, the catalyst can be filtered, washed with warm ethanol, and reused without decrease in activity.
Previously, we have synthesized a number of heterocyclic compounds13-18. Although numerous methods are capable of affecting these synthesis has been previously reported. Zn [(L)proline]2 has been used previously as a catalyst for synthesis of organic compound19.
The comparison of IR spectra shows That in IR L- prolin spectra seeing NH And OH spectra . and was deleted Thes spectra in Fe [ L- proline ]2 Catalysors , and shows The Complex was formed between Fe and L – prolin , and in fact complex is similar To Zn [L- proline]2.
Therefore, we reported the development of an efficient, a facile method and green synthesis for 1, 4-DHPs by Fe [(L)proline]2 as catalyst (Scheme 1). There is Fe [(L)proline]2 as the catalyst were environmentally friendly, and easy separation.
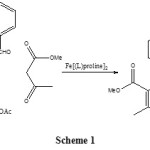 |
Scheme 1 Click here to View scheme |
General Procedure for the Preparation of the Fe [(L)proline]2
A mixture of Triethylamine (1 ml) and L-proline (4 mmol) in methanol (10 ml) was added. After solubilization with heat, reaction mixture was stirred for 10 min and ferrous sulfate (2 mmol) was added. A white precipitate was readily formed and after 1 hour it was collected by filtration to give the desired complex. IR spectra confirm formation of Fe [(L)proline]2, IR spectra fig 1 is for L-proline, IR spectra fig 2 is for ferrous sulfate and IR spectra fig 3 is for Fe [(L)proline]2. Comparison of the spectra shows that loss some of signals and picks is sign for formation complex of Fe [(L)proline]2.
 |
Figure 1 Click here to View figure |
 |
Figure 2 Click here to View figure |
 |
Figure 3 |
General Procedure for the Preparation of diethyl 2, 6-dimethyl-4-phenylpyridine-3, 5-dicarboxylate
A mixture of ethyl acetoacetate (2 mol), benzaldehyde (1 mol) and ammonium acetate (1 mol) and Fe [(L)proline]2 (% 10) in ethanol (20 ml was refluxed for 1.5 h. The obtained solid was filtered; the solid was washed with water and recrystallized using absolute ethanol.
Spectral data for diethyl 2, 6-dimethyl-4-phenylpyridine-3, 5-dicarboxylate
Yellow crystals, Yield 91%, IR (KBr, cm-1) ν: 3405, 3012, 2955, 1728. 1H NMR (400MHz, CDCl3) δ: 1.25 (t, 6H, 2CH3, J = 7. 4 Hz), 2.55 (s, 6H, 2CH3), 4.45 (q, 4H, 2CH2O, J = 7.4 Hz), 5.11 (s, 1H, CH), 7.23-7.80(m, 5H, Harom) 8.88 (s, 1H, NH).
Acknowledgement
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
References
- Visentin S., Rolando B., Di Stilo A., Frutterro R., Novara M., Carbone E., Roussel C., Vanthuyne N. and A. Gasco, J. Med. Chem., 47: 2688 (2004).
- Tsuruo T., Iida H., Nojiri M., Tsukagoshi S. and Sakurai Y., Cancer Res., 43: 2905 (1983).
- Malaise W.J. and Mathias P.C.F., Diabetologia., 28: 153 (1985).
- Poindexter G.S., Bruce M.A., Breitenbucher J.G., Higgins M.A., Sit S.Y., Romine J.L., Martin S.W., Ward S.A., McGovern R.T., Clarke W., Russell J. and Antal-Zimanyi I., Bioorg. Med. Chem., 12: 507 (2004).
- Krauze A., Germane S., Eberlins O., Sturms I. and Klusa V., Duburs G., Eur. J. Med. Chem., 34: 301 (1999).
- Koukabi N., Kolvari E., Khazaei A., Zolfigol M.A., Shirmardi-Shaghasemi B., and Khavasi H.R., Chem. Commun., 47: 9230 (2011).
- Ladani N.K., Mungra D.C., Patel M.P., and Patel R.G., Chin. Chem. Lett., 22: 1407 (2011).
- Saini A., Kumar S., and Sandhu J.S., J. Sci. Ind. Res., 67: 95 (2008).
- Bhatti R.S., Krishan P., Suresh, and Sandhu, J.S., J. Indian Chem. Soc., 87: 707 (2010).
- Vijesh A.M., Isloor A.M., Peethambar S.K., Shivananda K.N., Arulmoli T., and Isloor, N.A., Eur. J. Med. Chem., 46: 5591 (2011).
- Kolvari E., Zolfigol M.A., Koukabi N., and Shirmardi-Shaghasemi B., Chem. Papers., 65: 898 (2011).
- Long, S., Panunzio, M., Petroli, A., Qin, W., and Xia Z., Synthesis., 7: 1071 (2011).
- Azizian J., Hatamjafari F., Karimi A. R. and Shaabanzadeh M., Synthesis. 5: 765 (2006).
- Azizian J., Shaabanzadeh M., Hatamjafari F. and Mohammadizadeh M.R., Arkivoc., (xi): 47 (2006).
- Hatamjafari F., Synthetic Communications., ý 36: 3563 (2006).
- Azizian J., Hatamjafari F. and Karimi A. R., Journal of Heterocyclic Chemistry.ý, 43: 1349 (2006).
- Hatamjafari F. and Montazeri N., Turkish Journal of Chemistry.ý, 33: 797 (2009).
- Hatamjafari F., Orient. J. Chem., 28: 141 (2012).
- Rajender Reddya K., Rajasekhara C. V., and Gopi G., Synthetic Communications.,ý 37: 1971 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.









