Synthesis and Evaluation of Some New Pyrazoline Derivatives as Antimicrobial Agents
M. M. Kendre and M. A. Baseer*
Organic Chemistry Research Laboratory, Yeshwant Mahavidyalaya, Nanded - 431 602, India.
Biologically active Pyrazoline derivatives were efficiently synthesized in excellent yields and in less reaction time using ethanol via cyclization reaction of chalcones and hydrazine hydrate. These newly synthesized compounds were screened for their antimicrobial potencies which reflects moderate to good activity against different strains of bacteria and fungi employed. All the synthesized compounds were confirmed by IR, 1HNMR and Mass spectral data.
KEYWORDS:Chalcones; Hydrazine hydrate; Pyrazolines; Antimicrobial activities
Download this article as:| Copy the following to cite this article: Kendre M. M, Baseer M. A. Synthesis and Evaluation of Some New Pyrazoline Derivatives as Antimicrobial Agents. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Kendre M. M, Baseer M. A. Synthesis and Evaluation of Some New Pyrazoline Derivatives as Antimicrobial Agents. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25167 |
Introduction
Heterocyclic nitrogenous compounds and their fused analogues represent an important class of heterocyclic compounds. They exist in numerous natural products, display a wide range of biological and pharmaceutical activities. Pyrazolines are well known important nitrogen containing five membered heterocyclic compounds. Pyrazolines have received considerable attention in recent years due to their wide range of Antimicrobial1-4, Antiviral5, Antiosxidant6, Antidepressant7-8, Anticonvulsant9, Antiamoebic, Cytotoxic10, Antihistaminic11-12, Molluscicidal13, Anti-Inflammatory14, Anticancer15, Analgesic16, COX-2 inhibitor17-18, Antimalarial19, Anti-ubercular20, Aninociceptive21 activities. In addition, pyrazolines are used in the treatment of Parkinson’s, Alzheimers disease and cerebral edema22. Pyrazolines are also used as synthones for organic syntheses23-24. The present work describes the synthesis of new pyrazolines and their antimicrobial activities.
Material and Methods
Experimental
All the melting points were determined in open capillary method and are uncorrected. IR spectra were recorded in KBr spectrometer. 1HNMR spectra on a Avance 300 MHz spectrometer with DMSO as a solvent and TMS internal standard chemical shift, the chemical shift values are expressed in part per million (ppm) downfield from the internal standard and signals are quoted as s (singlet), d (doublet), T (triplet) and m (multiplet). Purity of the compounds is checked by TLC plates using benzene and ethyl acetate as an eluent in the ratio of (7:3 v/v).
General procedure for synthesis of Pyrazolines
A mixture of chalcone (0.001mol) and hydrazine hydrate (0.002mol) in 10 ml ethanol was reflux for 3 hrs. After completion of reaction (monitored by TLC) the reaction mixture was distilled off to remove the excess solvent and then it was poured into crushed ice. The solid obtained was washed with water and recrystllised from ethanol.
Scheme-I
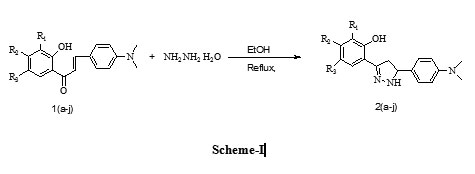
Table 1: Physical data of synthesized Pyrazoline derivatives (2a-j)
|
Sr.No. |
Entry |
R1 |
R2 |
R3 |
Molecular Formula |
Yield (%) |
M.P.0C |
|
1 |
2a |
Cl |
H |
Cl |
C17H17OCl2N3 |
92 |
152 |
|
2 |
2b |
I |
H |
Cl |
C17H17OClIN3 |
90 |
85 |
|
3 |
2c |
Br |
H |
Cl |
C17H17OBrClN3 |
88 |
149 |
|
4 |
2d |
Br |
H |
Br |
C17H17OBr2N3 |
86 |
166 |
|
5 |
2e |
H |
H |
Br |
C17H18OBrN3 |
90 |
160 |
|
6 |
2f |
H |
CH3 |
Cl |
C18H20OClN3 |
86 |
127 |
|
7 |
2g |
Br |
CH3 |
Cl |
C18H19OBrClN3 |
88 |
136 |
|
8 |
2h |
I |
CH3 |
Cl |
C18H19O IClN3 |
90 |
189 |
|
9 |
2i |
Br |
H |
CH3 |
C18H19OBrN3 |
92 |
198 |
|
10 |
2j |
I |
H |
Br |
C17H17OBrIN3 |
85 |
107 |
Results and discussions
A series of some novel 3-(subst-2-hydroxy-phenyl)-5-(4’-(dimethylamino-phenyl)-4, 5-dihydro-1H-pyrazole (pyrazoline) derivatives were synthesized by refluxing 3-(4’-Dimethylamino-phenyl)-1-(2-hydroxy-phenyl)-propenone (chalcone) derivatives and hydrazine hydrate. The uses of different chalcone for the synthesis of pyrazoline have been investigated. The presence of bromo, chloro, hydroxyl, iodo and methyl groups in different position of benzene ring of the chalcones and the use of hydrazine hydrate resulted in synthesis of new pyrazoline derivatives with significantly high yield.
Initially, the reaction condition was optimized by the investigation of model reaction of hydrazine hydrate and 3-(4’-Dimethylamino-phenyl)-1-(2-hydroxy-phenyl)-propenone(1a) in ethanol solvent at reflux temperature to obtain desired product (2a)(Scheme 1). With same reaction condition, several substituted Chalcones(1b-j) were treated with hydrazine hydrate and the results are summarized in Table 1. The structures of all the compounds were established from IR, 1HNMR and mass analysis. The 1HNMR spectra of 2a, 2c and 2d showed a characteristic peak at δ 3.0 (dd, 1H, HA), δ 3.53 (dd, 1H, HB), δ 4.8 (t, 1H, HX ).
All the compounds screened for antibacterial activity were also studied for antifungal activity against the selected strains. The compounds 2e, 3f and 2g showed moderate activity, while 2a, 2b and 2h showed significant activity in comparison with standard drug. The presence of pyrazoline moiety, substituents particularly having bromo, chloro, hydroxyl, iodo and methyl groups in the ring may be responsible for antimicrobial activity of this class of compounds.
Spectroscopic data of synthesized compounds
3-(3, 5-Dichloro-2-hydroxy-phenyl)-5-(4’-(dimethylamino-phenyl)-4, 5-dihydro-1H-pyrazole (2a)
IR (KBr): 1608 cm-I (C=N), 3333 cm-I (Ar-OH), 3421cm-I (N-H), 1226cm-I (C-N);
1HNMR (CDCl3): δ 2.85 (s ,6H, 2CH3), δ 3.0 (dd, 1H, HA), δ 3.53 (dd, 1H, HB), δ 4.8 (t, 1H, HX), δ 6.7-7.5 (m, 6H ,Ar-H), δ 8.0 (s, 1H, NH), δ 12.0 (s, 1H, OH),
M.S. (m/z): m=349, m+z = 351
3-(5-Bromo-2-hydroxy-phenyl)-5-(4’-(dimethylamino-phenyl)-4, 5-dihydro-1H-pyrazole (2c)
IR (KBr): 1608 cm-I (C=N), 3325 cm-I (Ar-OH), 3421 cm-I (N-H), 1234 cm-I (C-N);
1H NMR (CDCL3): δ 2.85 (s, 6H, 2CH3), δ 3.0 (dd, 1H, HA ), δ 3.52 (dd, 1H, HB), δ4.8 (t, 1H, HX), δ 6.7-7.5 (m,6H, Ar-H), δ 8.05 (s, 1H, NH), δ 12.1 (s, 1H, OH),
M.S. (m/z): m+1 = 395
3-(3, 5-Dibromo-2-hydroxy-phenyl)-5-(4’-(dimethylamino-phenyl)-4, 5-dihydro-1H-pyrazole(2d)
IR(KBr): 1609 cm-I (C=N), 3328 cm-I (Ar-OH), 3424 cm-I (N-H), 1230 cm-I (C-N);
1H NMR (CDCl3): δ 2.84 (s, 6H, 2CH3), δ 3.0 (dd, 1H, HA), δ 3.53 (dd, 1H, HB), δ 4.8 (t, 1H, HX ), δ 6.7-7.6 (m, 6H, Ar-H), δ 8.05 (s,1H, NH), δ 12.1 (s, 1H, OH),
M.S. (m/z): m+1=440, m+2 = 441
Antimicrobial Activity
For establishment of antimicrobial activity of the synthesized compounds, we utilized the reported Cup Plate method25. The experiment is performed at a concentration of 100µg/ml, we checked the activity of these molecules against different strains of bacteria and fungi as mentioned in Table 2. DMSO was used as solvent control. The obtained data of activity of all these tested compounds is shown in Table 2.
Table 2: Antimicrobial activityof synthesized pyrazoline derivatives (2a-j).
|
|
Bacteria (Zone of inhibition in mm) |
Fungi (Zone of inhibition in mm) |
|||||||||||
|
Entry |
A |
B |
C |
D |
E |
F |
G |
||||||
|
2a |
13 |
17 |
21 |
16 |
-ve |
RG |
-ve |
-ve |
|||||
|
2b |
16 |
13 |
22 |
12 |
RG |
RG |
RG |
RG |
|||||
|
2c |
— |
21 |
18 |
14 |
RG |
-ve |
RG |
RG |
|||||
|
2d |
— |
15 |
20 |
17 |
RG |
-ve |
-ve |
-ve |
|||||
|
2e |
12 |
— |
— |
12 |
RG |
-ve |
-ve |
-ve |
|||||
|
2f |
13 |
— |
21 |
— |
RG |
-ve |
RG |
RG |
|||||
|
2g |
16 |
14 |
— |
— |
RG |
RG |
RG |
RG |
|||||
|
2h |
14 |
17 |
17 |
16 |
-ve |
RG |
RG |
RG |
|||||
|
2i |
16 |
12 |
22 |
— |
RG |
RG |
-ve |
-ve |
|||||
|
2j |
12 |
18 |
21 |
— |
-ve |
-ve |
RG |
-ve |
|||||
|
Penicillin |
30 |
28 |
30 |
32 |
|||||||||
(Zone of Inhibition in mm)
A= Escherichia coli, B=Salmonella typhi, C= Staphylococcus aureus,
D=Bacillus subtilis E= Aspergillusniger F=penicilliumchrysogenum,
G=Fusariummoneliforme, H= Aspergillusflavus
–= No Antibacterial activity, RG= Reduced Growth (Moderate Activity)
-ve = Growth (Antifungal Activity Observed)
Ackowledgement
The authors are thankful to the Principal Yeshwant Mahavidyalaya, Nanded for providing all necessary research facilities to carry out this work. The authors are also thankful to Director IICT Hyderabad for providing spectral analysis facilities for the research work.
Reference
- Jadhav S. B., Shastri R. A., Gaikwad K. V. and Gaikwad S. V., E. J. Chem.,6(SI), S183-S188(2009).
- Patel B. N., Patel P. S. and Patel V. G., J. Chem. Pharm. Res., 3(2), 308-312(2011).
- Patel M. R., Dodiya B. L., Ghetiya R. M., Joshi K. A., Vekariya P. B., Bapodara A. H. and Joshi H. S., Int. J. Chem. Tech.Res., 3(2), 967-974(2011).
- Saundane A. R., JaishreeBadiger and P. M. Veeresha Sharma, Ind. J. Heterocycl. Chem., 14, 331-334(2005).
- Osama I. EI-Sabbagh, Mohamed M. Baraka, Samy M. Ibrahim, Christophe Pannecouque, Graciela Andrei, RoberSnoeck, Jan Balzarini, Adel A. Rashad, Eur. J. Med. Chem., 44, 3746-3753(2009).
- Kunal Hazra, L. V. G. Nargund, P. Rashmi, J. N. NarendraSharath Chandra and B. Nandha, Der Chemica Sinica, 2(2), 149-157(2011).
- Erhan Palaska, MutluAytemir, I. TayfunUzbay, DilekErol, Eur. J. Med. Chem., 36, 539-543(2001).
- Ruhoglu O., Ozdemir Z., Calis U.,Gumusel B and Bilgin A. A., Arzueim Foresch, 55 (8), 431(2005).
- Anees A. Siddiqui, Md. AzizurRahman, Md. Shaharyar, Ravinesh Mishra, Chem. Sci. J., CSJ-8, 1-10(2010).
- Faisal Haya, Aar Salahuddin, Sadiq Umar, Amir Azam, Eur. J. Med. Chem., 45, 4669-4675(2010).
- Rahaman S. A., Y. Ragjendra Prasad, K. Bhuvaneswasi, Phani Kumar, Int. J. Chem. Tech. Res., 2(1), 16-20(2010).
- Ch. Sridevi, K. Balaji, A. Naidu, S. Kavimani, D. Venkappayya and R. Suthakaran, Rasayan J. Chem., 1(2), 306-314(2008).
- M. F. EI Shehry, R. H. Swellem, Sh. M. Abu-Bakr, E. M. Ei-Telbani, Eur. J. Med. Chem., 45, 4783-4787(2010).
- PankajMalhora, ShashikantPaan, Anna PratimaNikalje, Int. J. Pharm. Pharm. Sci., 2(2), 21-26(2010).
- Anny Mahew, Mary Sheeja T. L., Arun Kumar T., K. Radha, H. J. D. Med., 3(2), 48-56(2011).
- Mohd Amir and Shikha Kumar, Ind. J. Chem., 44B, 2532-2537(2005).
- RossellaFioravani, Adriana Bolasco, Fedele Manna, Francesca Rossi, Francisco Orallo, Francesco Oruso, Stefano Alcaro, Roberto Cirilli, Eur. J. Med. Chem., 45, 6135-6138(2010).
- Norris , Colon-Cruz R and Ripin D. H., Org. Biomol .Chem., 3, 1844(2007).
- Badri Narayan Acharya, DeepikaSaraswant, Mugdha Tiwari, Asish Kumar Shrivastava, Ramarao Ghorpade, SarojBapna, Mahabir Parshad Kaushik, Eur. J. Med. Chem., 45, 430-438(2010).
- Mohamed Ashraf Ali, Mohammad Shaharyar, Anees Ahamed Siddiqui, Eur. J. Med. Chem., 42, 268-275(2007).
- Zafer Asim Kaplancikli, GulhanTuran-Zitouni, Ahmet Ozdemir, Ozgur Devrim Can, Pierre Chevallet, Eur. J. Med. Chem., 44, 2606-2610 (2009).
- Kawazura H., akahashi Y., Shiga Y., Shimada F.,Ohto N.T A., Jpn. J. Pharmacol,73(4), 317(1997).
- Padmavathi V., Sumathi R. P., Chandrasekhar B. N and Bhaskarreddy D., .J Chem. Res., 610(1999).
- Bhaskarredy D.,Chadrasekhar B. N., Padmavathi V and Sumathi R. P., Synthesis, 491(1998).
- Barry A. L., The Antimicrobial Susceptibility Test: Principle and Practices, Edited by Illus Lea and Febiger, Philadelphia, P. A.(USA), 180(1976).

This work is licensed under a Creative Commons Attribution 4.0 International License.









