Synthesis and Characterization of Novel Amphiphilic Block Copolymer Poly(4-Vinyl Benzene Chloride)-B-Poly(Ethylene Oxide)
N. Nemiche, F.Z. Sebba and S.Ould-Kada
Department of Chemistry, Faculty of Science, Islamic Azad University-Tonekabon Branch, Tonekabon, Iran.
Article Received on :
Article Accepted on :
Article Published : 19 Oct 2016
We introduce Cu [(L)proline]2 as a Novel Catalyst, cheap, environmentally friendly, and easy separation for Synthesis of 1, 4-Dihydropyridines under solvent-free condition. IR spectra Confirms formation complex of Cu [(L)proline]2.
KEYWORDS:1; 4-dihydropyridines; solvent-free; Cu [(L) proline]2
Download this article as:| Copy the following to cite this article: Nemiche N, Sebba F. Z, Ould-Kada S. Synthesis And Characterization Of Novel Amphiphilic Block Copolymer Poly(4-Vinyl Benzene Chloride)-B-Poly(Ethylene Oxide). Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Nemiche N, Sebba F. Z, Ould-Kada S. Synthesis And Characterization Of Novel Amphiphilic Block Copolymer Poly(4-Vinyl Benzene Chloride)-B-Poly(Ethylene Oxide). Available from: http://www.orientjchem.org/?p=22564 |
Introduction
Amphiphilic block copolymers consist of both hydrophobic and hydrophilic chain segments combined in a single macromolecule and are typically found to aggregate and adsorb at surfaces, similar to low molecular weight surfactants 1. The phase behavior of such system can show a high degree of richness and complexity, such as the formation of large variety of lyotropic liquid crystalline phases at higher block copolymer concentrations and in the presence of a single selective solvent or two immiscible selective solvents2, and the generation of a variety of supermolecular structures in aqueous solution such as spherical or rodlike micelles, vesicles, fibers, network structures, and lamellar or helical aggregates 3. These copolymers have received a lot of attention because of their application as gel-formers, surface modifiers, foam andcolloid stabilizers, thickeners, wetting agents, compatibilizers, microreactors and nanostructure materials 4.
Recently a special focus is placed on the use of self assembled block copolymer in pharmaceutical and biomedical applications 5.In most cases the hydrophobic groups are either alkyl chains, from octyl to octadecyl, or contain an aromatic ring (phenyl, naphthyl, pyrenyl, etc.).
Extensive investigations have been conducted on amphiphilic cellulose 6, PEG 7, poly(acrylic acid) 8, and others 9,aswellason polyacrylamide based copolymers 10. The former polymers were mainly prepared by chemical modification of a preformed polymer, and the latter were obtained by copolymerization of the appropriate monomers.. Generally, the hydrophilic block is made of poly(ethylene oxide)(PEO), because of its availability, high solubility in water, and high biocompatibility 11-15. PVBC is extensively used as hydrophobic segment in amphiphilic block copolymers, since it is highly hydrophobic and crystalline polyester, and a suitable component of micelle inner core that favours the loading of hydrophobic drugs.
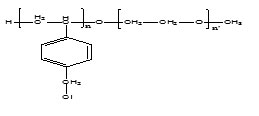
In the framework of our continuing interest in copolymeric materials for biomedical applications16–20 ,we report here the synthesis and the characterization of amphiphilc copolymers poly (VBC)-b-poly(EO), the characterization and the micellization of the copolymers obrained was investigated in detail with a FTIR, 1H NMR, GPC methods. In particular, tensiometer to determinate the CMC and the atomic force microscopy (AFM) and SAXS was also used to study the microscopic morphologies within those micelle formed. Schema 1 represented the structure of the amphiphilic block copolymer poly(4-Vinylbenzenechloride)-b-poly(ethylene oxide).
Schema1. The structure of amphiphilic block copolymer poly(4-Vinylbenzenechloride)-b-poly( ethylene oxide).
Experimental Part
Materials
All commercial products were purchased from Aldrich and used as received, unless otherwise stated. Solvents were distilled before use. Poly(ethylene oxide) PEO and 4-chloride vinylbenzene (VBC) were distilled under reduced pressure.
Characterization
FTIR measurements
FT-IR spectra were obtained on a Nicolat Avatar 320FT-IR spectrometer, 32 scans at a resolution of 1cm-1 were collected with a KBr disk at room temperature in the range of 4000 – 500 cm -1 (Algeria). (Fig.1).
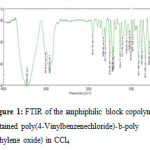 |
Figure 1: FTIR of the amphiphilic block copolymer obtained poly(4-Vinylbenzenechloride)-b-poly(ethylene oxide) in CCl4.
|
1H NMR measurements
The 1H NMR spectra were recorded on Bruker AM400 (400MHZ) spectrometer. (French) in CDCl3. High-resolution solid state experiments were carried out at 25°C using a Bruker MSL-400 spectrometer operating, using the crosspolarization/magic angle spinning(CP/MAS) probe with 4 mm O.D. rotors , under high power proton decoupling. The sample spinning is performed at 7.5 KHz, to easily recognize the side bands. For each spectrum, 1000 scans are averaged using a recycling time of 4 s. (Fig. 2).
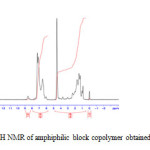 |
Figure 2: 1H NMR of amphiphilic block copolymer obtained in CDCl3 |
GPC measurements
The average molecular weights, and the polydispersity index PI were determined from GPC(Gel Permeation Chromatography ) using a high pressure liquid chromatography pump with HP 1050 system equipped with a vacuum degasser, a refractive index detector. The eluting solvent was tetrahydrofuran (THF) .The molecular weight calibration curve was obtained using polystyrene standards. (Fig. 3).
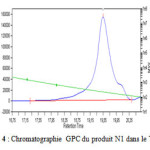 |
Figure 3: Chromatographie GPC du produit N1 dans le THF |
Differential scanning calorimetry
DSC measurements were carried out using a Perkin Elmer DSC pyris 1 calibrated with indium and cyclohexane. Each sample was scanned with a heating rate of 10 °C min–1. Four scans were performed on each sample. The measured DSC data are averaged on three last subsequent scans. The glass transition temperature Tg was determined by standard extrapolating the linear portion of DSC traces. (Fig. 4).
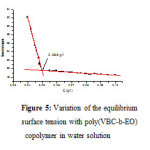 |
Figure 4: Variation of the equilibrium surface tension with poly(VBC-b-EO) copolymer in water solution.
|
AFM measurements
Samples for AFM measurements were prepared as given below: a drop of copolymer solution pre-pared by dissolving the star block copolymers directly in double distilled water was dropped on a freshly cleaved mica substrate, and the mica was rapidly frozen; then the frozen micellar solution on a mica wafer was lyophilized for 48 h to remove water. The AFM images were recorded with a Nanoscope III from Digital Instruments operated in the tapping mode in air using microfabricated Si (type NCH) cantilevers with a spring constant between 27 and 53 N m-1, resonance frequency in the range of 301–365 kHz, and 1 and 2 Hz scanning speed. (Fig. 6).
 |
Figure 5: AFM image of a block copolymer poly (VBC–b–EO) Cylindrical Morphology
|
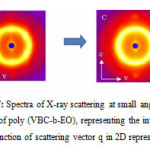 |
Figure 6: Spectra of X-ray scattering at small angles of the film of poly (VBC-b-EO), representing the intensity I as a function of scattering vector q in 2D representation.
|
DRX (SAXS)
SAXS analyzes were performed at the Research Centre Paul Pascal in Bordeaux, in collaboration with Virginia Ponsinet, on mounting Nanostar (Brucker). It includes a copper anode tube has an operating voltage of 40 kV and a current of 35 mA. Optics consists of two mirrors Gobels select the Kα line (λ = 1.54 A) of copper. The beam is collimated by three holes, “Pinholes”. A detector to son “Histar” Bruker dimensions of 22 x 22 cm is placed at a distance D of the sample and allows acquiring a two-dimensional spectrum which is then integrated. This gives the scattered intensity I (q) in arbitrary units as a function of scattering vector q. The range of scattering vectors available ranges from 0.0096 to 0.2 A-1 to D = 1 m. Analyses were performed on samples in the form of films and powders. The films are placed directly on the sample holder, the powders obtained by freeze-drying, are contained in sealed glass capillary Lindman of 1mm thick.
Synthesis of amphiphilic block copolymer
The synthesis of the amphiphilic block copolymers was made by using the direct method, wich consist with the starting of 4-Vinyl benzene chloride (VBC)(6,44mol/l) with H2SO4 (0,01mol/l) as initiator, follow-up of deactivation of the macrocation obtained by poly(ethylene oxide) (5,13mol/l), who gave place to the copolymer block formation poly(4-Vinylbenzenechloride)-b-poly(ethylene oxide).
Characterization of amphiphilic block copolymer
FT-IR (cast film): 2920,6 cm-1 (γ CH), 1423cm-1 (d CH), and 754 cm-1 (γ C-Cl) , 1105,9 cm-1 (γ C-O-C)(Fig.1)
Figure 1: FTIR of the amphiphilic block copolymer obtained poly(4-Vinylbenzenechloride)-b-poly(ethylene oxide) in CCl4 1H NMR (CDCl3): d = 3.7ppm (CHCH2), 2.0ppm (CH2CH2C-O), 1.7ppm (CH2CH) and 1.1 ppm (CCH3) , 7.26ppm (CH benzene), 2.05ppm(CH2Cl).(fig.2)
The average molecular weights, and the polydisepersity index PI were determined from GPC(Gel Permeation Chromatography ) .The molecular weight calibration curve was obtained using polystryrene standards. The values of Mn =1393, Mw= 1482 and PI=1,062 .(fig.3)
The measures of the tension relative superficial in the aqueous solution of copolymer block show that there is a reduction in the tension superficial of the water.
We were able to, determined a micellaire critical concentration wich equal 0,096mg/ml (Fig.5).
These tensioactive properties allow us to conclude that this copolymer establish a new class of tensioactive no-ionic.
To confirm the structure of the poly (VBC-b-EO) micelle in aqueous solution the sample was examined by AFM and by DRX 2D. In this study thin deposit on mica was prepared by in situ freeze-drying procedure from solution.
Synthesis and characterization of amphiphilic block copolymer were carried out, in order to obtain materials likely to have potential applications in various fields and in particular with the aim of preserving the environment. The analysis by 1H NMR, FTIR and GPC confirmed the sequence copolymer of poly (4-vinyl benzene chloride-b- ethylene oxide) obtained and the results obtained open a direct routs for the preparation of a series of amphiphilic block copolymer with sequences of poly (4-vinyl benzene chloride), and of poly(EO). The tensioactive properties allow us to conclude that this amphiphilic block copolymer establish a new class of tensioactive no-ionic. The morphology of this amphiphilic copolymer is cylindrical.
Acknowledgements
This work was performed in the University of Oran in the laboratory of Chemical physical Macromolecular. The co-authors wish to acknowledge Dr. F.Z.SEBBA and Pr .S.OULD KADA for helping and CNRS of Marseille and Grenoble for helping with NMR and GPC measurements and to Mullhose and Grenoble for helping with AFM and DRX ( SAXS) measurements.
References:
- Zhang S. Nat Biotechnol .21, 1171 (2003)
- Roesler A, Vandermeulen GWM, Klok HA. Adv Drug Deliv Rev. 53,95 (2001)
- Cannizzaro SM, Padera RF, Langer R, Rogers RA, Black FE,Davies MC, et al. Biotechnol Bioeng .58,529 (1998)
- Zoli Mikoczy , Håkan Tinnerberg , Jonas Björk ,Maria Albin. Cancer Incidence and Mortality in Swedish Sterilant Workers Exposed to Ethylene Oxide: Updated Cohort Study Findings 1972–2006, Int. J. Environ. Res. Public Health. 8, 2009(2011)
- Chien, Y.C.; Liu, H.H.; Lin, Y.C.; Su, P.C.; Li, L.H.; Chang, C.P.; Tang, D.T.; Chen, C.Y. Ethylene oxide sterilization in the medical-supply manufacturing industry: assessment and control of worker exposure. J. Biomed. Mater. Res. B Appl. Biomater. 83, 527(2007)
- Haufroid, V.; Merz, B.; Hofmann, A.; Tschopp, A.; Lison, D.; Hotz, P. Exposure to ethylene oxide in hospitals: biological monitoring and influence of glutathione S-transferase and epoxide hydrolase polymorphisms. Cancer Epidemiol. Biomarkers Prev. 16, 796(2007)
- Yomg, L.C.; Schulte, P.A.; Kao, C.Y.; Giese, R.W.; Boeniger, M.F.; Strauss, G.H.; Petersen, M.R.; Wiencke, J.K. DNA adducts in granulocytes of hospital workers exposed to ethylene oxide. Am. J. Ind. Med. 50, 293(2007)
- Xelegati, R.; Robazzi, M.L.; Marziale, M.H.; Haas, V.J. Chemical occupational risks identified by nurses in a hospital environment. Rev. Lat. Am. Enfermagem. 14, 214(2006)
- Bizzarri R, Solaro R, Talamelli P, Chiellini E. Synthesis and characterization of new poly(ester-amide)s containing oligo(oxyethylene) segments. J Bioact Comp Polym.15,43(2000)
- Chiellini E, Chiellini EE, Chiellini F, Solaro R. Targeted administration of proteic drugs I preparation of polymeric nanoparticles. J Bioact Compat Polym,16,441(2001)
- Bizzarri R, Chiellini F, Solaro R, Chiellini E, Cammas- Marion S, Guerin P. Synthesis and characterization of new malolactonate polymers and copolymers for biomedical applications. Macromolecules.35,1215(2002)
- Bizzarri R, Chiellini F, Ober CK, Saltzman WM, Solaro R. Influence of structural parameters on the ring-opening polymerization of new alkyl malolactonate monomers and on the biocompatibily of polymers therefrom. Macromol
- Chem Phys. 203,1684(2002).
- Signori F, Solaro R, Chiellini E, Lips PAM, Dijkstra PJ, Feijen J. Synthesis and characterization of segmented poly(ether ester)s containing h-bonding units. Macromol Chem Phys.204,1971(2003).
- Signori F, Fiumi C, Bizzarri R, Chiellini F, Solaro R, Chiellini E. Nanospheres from amphiphilic graft copolymers for targeted protein release. J Controlled Release ,87,247(2003).
- Solaro R, Chiellini F, Signori F, Fiumi C, Bizzarri R, Chiellini E. Nanoparticle systems for the targeted release of active principles of proteic nature. J Mat Sci Mat Med.14,705(2003).
- Signori F, Chiellini F, Solaro R. New self-assembling biocompatible-biodegradable amphiphilic block copolymers.Polymer .46,9642(2005).
- Chiellini E, Chiellini EE, Chiellini F, Solaro R. Nanoparticles for targeted administration of proteic drugs surface properties and targeting propensity. J NanoSci Nanotech.6,3040(2006).
- Solaro R, Chiellini F. Nanoparticles for the targeted delivery of proteins and peptides. In: Ravi Kumar, editor. Handbook of particulate drug delivery. New York: American Scientific Publisher . Chap 10.( 2007)

This work is licensed under a Creative Commons Attribution 4.0 International License.









