Synthesis and Characterization of Copper Complexes of A Schiff Base Derived from 1H - Indole 2,3 Dione
P. C. Sandhya Rani, G. S. Thara* and Lekshmy R. K.
Department of Chemistry, University College, Thiruvananthapuram - 695 036, India.
A series of novel metal complexes of Schiff bases have been prepared by the interactions of Cu (II) with 1,3 - di hydro - 3 - [2-(Phenyl) – ethylidene]-2H-indol-2-one-hydrazine carboxamide in bimolecular ratios. All the new compounds have been characterized by elemental analysis, conductance measurement, magnetic moment determinations, IR, UV and NMR spectral studies and thermal studies. The physio-chemical studies and spectral data indicate that the ligand acts as a bidentate chelating ligand coordinating through nitrogen and oxygen atoms. Square planar geometry is proposed for all the complexes.
KEYWORDS:Schiff bases; Copper; Isatin
Download this article as:| Copy the following to cite this article: Rani P. C. S, Thara G. S, Lekshmy R. K. Synthesis and Characterization of Copper Complexes of A Schiff Base Derived from 1H - Indole 2,3 Dione. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Rani P. C. S, Thara G. S, Lekshmy R. K. Synthesis and Characterization of Copper Complexes of A Schiff Base Derived from 1H - Indole 2,3 Dione. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25199 |
Introduction
Although there is a wealth of information concerning transition metal complexes with isatin schiff bases, only few investigations have appeared in the literature to describe the metal complexes of schiff bases derived from isatin with acetophenone and semicarbazide. Metal complexes of Schiff bases have unusual magnetic properties and relevance to biological systems 1,2. They exhibit numerous biological activities such as anticancer, antitumour, antibacterial and antifungal 3,4,5. There are extensive uses for isatin and its derivatives and these are versatile reagents in organic synthesis to obtain heterocyclic compounds and as raw material for drugs. Metal complexes of semicarbazones have given considerable interest in view of their industrial and biological importance 6. Cu (II) complexes have aroused general interest in recent years by the hope of modelings of biological molecules that contain copper. In the present study synthesis and characterization of Cu (II) complexes of the Schiff bases 1,3 – dihydro-3-[2 – (Phenyl)-ethylidene] – 2H – indol -2 – one – hydrazine carboxamide was prepared by condensation of isatin with acetophenone and semicarbazide (IAS).
Experimental
Synthesis of Ligand
The ligand was prepared by the condensation of isatin and acetophenone in equimolar ratio and the resulting mixture was condensed with hydrazine carboxamine in presence of sodium acetate in 1:1 molar ratio in absolute alcohol. The reaction mixture was then refluxed over a water bath for 4-6 hours and allowed to stand overnight. The products were recrystallised from the solvent ethanol and dried in vacuum7. Physico-chemical properties of the ligand was determined and given in Table (1).
Structure of Ligand
 |
Scheme 1 Click here to View scheme |
Synthesis of Cu (II) complexes
All complexes except the thiocyanate complex were prepared by the following general method. Hot methanolic solution of metal salts were added to a hot methanolic solution of the ligand (0.002 mol) in 1:2 ratio. The reaction mixture was heated under reflux for about 4-5 hours in presence of few drops of conc. HCl. The resulting solution was concentrated to half of its volume and allowed to cool. On cooling the complexes separated out which are filtered and washed several times with aqueous methanol, diethyl ether and finally dried over P4 O10 in vacuo.
For the preparation of thiocyanate complex about 0.5g of NH4SCN was added to a methanolic solution of metal chloride (2 mmol) stirred well and filtered. The filtrate was then refluxed with a hot methanolic solution of ligand (2 mmol) for nearly five hours in a water bath. It is then concentrated and the solid formed was filtered, washed successively with aqueous ethanol, finally with diethyl ether and then dried over P4O10 in vacuo. All the chemicals and reagents were of analar grade [E. Merck and B.D.H (A. R)
Analytical methods and physical measurements
Chloride8, Sulphur8 and Copper9 was estimated by standard methods, Conductivity measurements were made with a Syntronics direct reading conductivity bridge. The magnetic susceptibilities were recorded at room temperature by the Gouy method. CHN analysis of the ligand and complexes were carried out on a vario EL – III CHN Elemental analyzer at the SAIF, Cochin University of Science and Technology. IR spectra of the ligand and their complexes were recorded with the help of Schimadzu IR – prestige spectrophotomer in KBr pellets at Govt. Womens College, Thiruvananthapuram. The electronic spectra were recorded on a Schimadzu UV – 2450 spectro photometer at Govt. Womens College, Thiruvananthapuram.
The 1H NMR spectra of the ligand IAS was recorded using a Brucker DRX-500 NMR spectro photometer using TMS as the internal standard at NIIST, Thiruvananthapuram. Thermal analysis was carried out using a Schimadzu DTG-60 differential thermogravimetric equipment. Kinetic parameters viz. Activation energy (E), Frequency factor (z) and entropy of activation (ΔS) were evaluated using the thermal analysis data.
Results and Discussion
All the complexes are deeply coloured, nonhydroscopic, stable in air and crystalline state. They are soluble in methanol and ethanol, partially soluble in benzene and insoluble in ether.
The IR spectrum of the free ligand exhibits two sharp bands at 3469 and 3340 cm-1 assaignable to asymmetric and symmetric vibrations of NH2 group which remain at almost the same positions in all the metal complexes suggests that the amino group is not involved in chelation. In the IR spectra of ligands a band at 3280 cm-1 assaigned to –NH stretching frequency of isatin moiety which remains unaltered in the complexes suggesting that –NH of isatin moiety is not involved in chelation. Similarly in the free ligand a band at 3151 cm-1 assaigned to –NH stretching frequency of semicarbozone remains unaltered almost in the metal complexes suggesting that –NH of semicarbozone is not involved in chelation. and observed at 1749 and 1624 cm-1 respectively in the ligand spectrum are shifted to ~1690 and ~1570 cm-1 in the spectra of the complexes suggesting the participation of these groups in coordination. Thus the ligand exhibit a neutral bidentate behavior in all the complexes. Also the IR spectra of the complexes show bands at 530 and 420 cm-1 which may be attributed to and respectively. In the I.R spectra of nitrate complex of IAS, three additional bands 1513, 1381 and 1060 cm-1 are seen which are not present in the spectra of the ligands are attributed to 5,1and 3modes of the co-ordinated nitrate ion. Since the difference between 5and 1is 132 cm-1 it is suggested that the nitrate ion is coordinated monodentately to the metal ion10. In the spectra of perchlorate complex of IAS two strong bands are observed at 1120 and 1034 cm-1. These can be assaigned to 4and 1of monodentate per chlorate. The bands found at 693 mc-1 and 660 cm-1 assaigned to 3 and 5of vibrations are characteristics of monodentately coordinated perchlorate group11, which is further supported by the molar conductance data. The thiocyanate complex shows very strong band at 2133 cm-1(C-N), medium intensity bands at 786 cm-1 (C-S), and 468 cm-1 (NCS) confirming N-coordinated nature of the thiocyanate group12.
1H NMR spectra of the free ligand exhibit a singlet at d 11.28 ppm due to the -NH proton, multiplets in the region d 7.70 ppm attributable to aromatic protons, singlet at d 3.45 ppm attributable to –NH2 group and singlet at d 11.94 ppm attribu table to –NH of the ring.
The electronic spectra of the ligand IAS exhibited an absorption band at 202 nm due to the p-p* transition of >C=0 group and at 271 nm due to n-p* transition occurred at 268nm and at 270nm. The n-p* transition undergoes a blue shift indicating that the lone pair electrons of oxygen are coordinated to the metal ion. The p-p* transition of >C=0 group of complexes are found at 213 and 210nm. The p-p* transition undergoes red shift with an increase in wavelength. Similarly the absorption bands seen at 322 and 389 are attributed to p-p* and n-p* transitions of >C = N group. Spectra of all complexes exhibit blue shift for n-p* transition which is observed at 387nm and 384nm respectively and a red Shift for the π – p* (325 nm and 326 nm) transition in all complexes, suggesting the co-ordination of nitrogen atom. In all complexes charge transition spectra occurred at the 420 nm and to 428nm. The electronic spectra of these complexes indicate square planar geometry 13,14 for all complexes.
Table 1: Analytical and physical data of ligand and complexes.
|
Species |
Colour |
Analysis % found (Cal) |
μeff |
^M
|
||||
|
M |
C |
N |
Cl |
S |
||||
|
IAS |
Bright Yellow |
– |
66.62 (66.6) |
18.3 (18.3) |
– |
– |
– |
– |
|
[Cu(IAS)(Cl2) |
Dark Blue |
14.4 (14.6) |
46.42 (46.41) |
10.39 (10.9) |
15.8 (15.83) |
– |
1.79 |
11.2 |
|
Cu (IAS) (NO3)2 |
Bluish Green |
11.56 (11.6) |
37.31 (37.25) |
15.23 (15.34) |
15.8 (15.83) |
– |
1.8 |
10.7 |
|
[Cu (IAS) ( ClO4)2 |
Bluish Green |
11.0 (11.19) |
35.85 (35.95) |
9.83 (9.87) |
12.3 (12.3) |
– |
1.82 |
9.1 |
|
[Cu (IAS) (NCS) (Cl)] |
Dark Blue |
13.6 (13.76) |
46.63 (46.69) |
15.41 (15.41) |
7.64 (7.68) |
6.88 (6.91) |
1.82 |
8.1 |
Magnetic moment in B.M and molar conductance using nitrobenzene in Ω¯1 cm2 mol ¯1x10 ¯3 M
The molar conductance values of the complexes exhibit a non-electrolytic nature for all the metal complexes. The magnetic moments of all the Cu (II)complexes under the present study are found to be in the range 1.79 to 1.83 B.M at room temperatures which suggests a square planar geometry around the metal ion15..
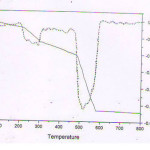 |
Figure 1 Click here to View figure |
 
TG and DTG curves of [Cu(IAS)Cl2]
The thermal decomposition behavior of the complex shows a plateau upto 2100C indicating the complex is stable up to this temperature and no co-ordinated water molecule is present. The TG curve of the complex shows two weight loss curves indicating that the complex has undergoes a two stage decomposition. The first stage of decomposition starts of 2100 C and completed at 3100 C. During the process there is a mass loss of 16% (15.8% is theoretical), which correspond to the elimination of the anonic part. The second decomposition stage range from 4800C-5800C which correspond to the elimination of the ligand part. At the end of these two stages, the anonic and the ligand part were completely removed and Copperoxide was obtained as the final residue. The percentage mass loss of 83.5% agrees with the theoretical value 82.8%. The energy of activation (E) for the first stage of decomposition is found to be 117.45 KJ mol -1 and that of second stage is 239.54 KJ mol -1 . Entropy of activation (Δs) for the first stage is found to be -127.105 and second stage is found to be -123.353. The negative value of Δs for the two stages of decomposition show that the activated complex has a more ordered structure than the reactants.
Conclusion
The analytical and spectroscopic datas suggest that all the metal complexes have the general formula (CuLX2) where L=IAS and x=Cl–, NO3¯,ClO4¯ except for the thiocyanate complex. The composition of thiocyanate complex is [(Cu L (NCS) (Cl)]. A square planar geometry has been tentatively proposed for all the complexes.
References
- L. Nathan, J.E. Koehne, J.M. Gilmore, K.A. Hannibal, W.E. Dewhirst and T.D. Mai, Polyhedron, 22, 887 (2003).
- P.R. Patel, B.T. Thakur and S. Zele, Indian J. Chem, Sect.A, 38, 563 (1999)
- Priyakumari, S. Prakash and D. Prakash, J. Indian Chem. Soc, 88, 1647 (2011)
- M.C.R Arguelles, S.M. Vazquez, P.T. Touceda, J.S. Matalobos, A.M.G. Deibe, M.G. Ferrari, G. Pelosi, C. Pielizzi and F. Zanni, J. Inorg. Biochem, 101, 138 (2007)
- P. Kamalakannan and D. Venkappayya, J. Inorg. Biochem, 21, 22(2002).
- S. Laly, G. Parameswaran, Asian. J. Chem. 3, 712 (1993)
- R.V. Singh, N. Fahmi and M.K. Biyala, J. Ira. Chem. Soc, 2, 40 (2005)
- A.I. Vogel A text book of quantitative inorganic analysis, 5th ed. ELBS. London, 1996.
- E. Kurz, G. Kober and M. Beri, Anal. Chem. 30, 1981 (1958)
- M.L. Harikumaran Nair and Ajnu. S. Appukuttan J. Indian. Chem.. soc, 89, 323 (2012)
- M.L. Harikumaran Nair and L. Shamla, J. Indi. Chem. Soc, 86, 913 (2009)
- M.L. Harikumaran Nair and. K.P. Lalitha, J. Indian Chem. Soc, 88, 323(2011)
- H.B. Gary, C.J. Ball housen, J. Am. Chem. Soc. 85, 260 (1963)
- J.L. Vats, S. Sharma, N.C. Gupta, H. Singh Synth. React. Inorg. Met. Org. chem.. 14, 521 (1984)
- J. Lewis and R.A. Walton, J. Am. Chem. Soc. 85, 1557 (1966)

This work is licensed under a Creative Commons Attribution 4.0 International License.









