Study of Photochemistry Without Light of Substituted Benzylidine Acenaphthenones
Vijender Goel
Department of Chemistry, Maharshi Dayanand University, Rohtak, India.
In order to draw a parallel between light induced reactions as well as photochemistry without light (chemiluminescence), the substituted benzylidineacenaphthenones were subjected to dioxetanedione reactions, which are formed in situ by the reaction of 2,4-dimitrophenyloxalate with hydrogen peroxide to provide chrysene derivatives as well as naphthalene 1,8-dicarboxylic acid anhydride and benzoic acid whose structures were determined by IR and 1H NMR spectra.
KEYWORDS:Acenaphthenone; benzylidineacenaphthenone; Methanochrysene; naphthalenedicarboxylic acid anhydride; photochemistry without light
Download this article as:| Copy the following to cite this article: Goel V. Study of Photochemistry Without Light of Substituted Benzylidine Acenaphthenones. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Goel V. Study of Photochemistry Without Light of Substituted Benzylidine Acenaphthenones. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25191 |
Introduction
An intriguing and potentially useful development of the mechanistic studies on chemiluminescence has been the realization that thermal decomposition of highly strained molecules provides, a useful source of excited state compounds without the need for a light source.1-3 Instead of electronic excitation energy being lost by light emission as in chemiluminescence, intramolecular energy transfer results in excited states of acceptor molecules which usually yield the same chemical products as those achieved by direct irradiation of the acceptor. First example of ‘photochemistry without light’ was described by E.H. White and coworkers.1 The decomposition of dioxetanedione also affected certain transformations4. The dioxetanedione decomposes following Woodward Hoffman rule5 to give one molecule of carbon dioxide in the excited state which transfers its energy to the substrate raising it to its excited state. The dioxetane-lanthanide complex energy transfer has been reported.6,7
Discussion
1-Acenaphthenone8 (1) was reacted with benzaldehyde in the presence of alcoholic potassium hydroxide at 0-5°C to obtain benzylidineacenaphthenone (2). Its structure was established by its IR9 and 90 MHz 1H NMR spectra.10 In a similar fashion o-chlorobenzaldehyde was reacted with 1-acenaphthenone to obtain o-chlorobenzylidine-acenaphthenone (3) whose structure was also confirmed by its IR and 90 MHz 1H NMR spectra. The benzylidineacenaphthenone (2) was then taken in ethanol and 2,4-dinitrophenyloxalate was added in equimolecular quantities. Gradual addition of 30% hydrogen peroxide (50 ml) to this reaction mixture exhibited chemiluminescence as indicated by emission of light. Usual work up of reaction mixture provided 10(H)oxo–10, 11-methanochrysene (4) in addition to 1,8-naphthalenedicarboxylic acid anhydride (5). The rest of benzylidine–acenaphthenone (2) was recovered unreacted. The formation of chrysene derivative (4) was confirmed by its mass spectrum, however its NMR could not be recorded properly due to its poor solubility. The structure of acid anhydride (5) was confirmed by its IR, 1H NMR and mass spectra.
In a similar fashion, o-chlorobenzylidineacenaphthenone (3) was subjected to photolysis without light as mentioned above which gave 10(H)oxo-10,11-methano-1-chlorochrysene (6) accompanied by 1,8-naphthalenedicarboxylic acid anhydride (5) in addition to unreacted starting material. The structure of chrysene derivative was confirmed by mass spectrum showing isotopic cluster due to chlorine atom in the molecule while the structure of anhydride (5) was confirmed by its m.p. and mixed m.p. with sample mentioned above.
When N-nitrosodiphenylamines, N-benzoylphenyl hydrazones and C-alkylnitroso derivatives were treated with hydrogen peroxide, exhaustive oxidation products were observed and therefore photochemistry without light could not be attempted on this class of compounds.
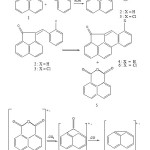 |
Scheme 1 Click here to View scheme |
 
Result
This method is extremely useful in those cases where hydrogen peroxide does not react with the substrate. In the cases where it reacts with the substrate, the method does not work. Advantage of this method is that it incorporates advantage of sensitised reaction as well as photochemistry using a monochromatic light and gives neat reaction products. The products were exactly similar to those reproted11 except that no tar formation could be observed.
Experimental
Melting points were determined in open capillary tubes and are uncorrected. IR spectra were recorded on Perkin-Elmer Spectrum BX-series FTIR, 1H NMR spectra on Bruker Avance II 400 MHz NMR spectrometer using tetramethylsilane as internal standard and chemical shift values are reported in δ scale. Mass spectra were obtained on a Jeol JMSD-300 spectrometer at 70 ev.
Benzylidineacenaphthenone (2)
Acenaphthenone (I; 3.36 g; 0.02 mole) and benzaldehyde (2.12g; 0.2mol) in ethyl alcohol (25 ml) was taken in a R.B. flask (100 ml) and reaction mixture was cooled to 0° in an ice salt bath. Sodium hydroxide (2.0g) in ethyl alcohol (18.0 ml) was added to stirred reaction mixture. Stirring was continued for an additional hr and then the contents were diluted with cold water. The separated solid was filtered, washed with excess water and recrystallised from ethanol/pet ether to procure 4.0 g (77.3%) of light yellow coloured crystals, m.p. 122-23°. IR (KBr): 3020 (-CH aromatics), 1700 (>C=O) and 1625 cm-1 (C=c). 1H NMR (CDCl3 + TFA): 7.90 (1H, s, benzal proton) and 8.15 to 7.50 (11H, m, rest of aromatic protons).
O-chlorobenzylidineacenaphthenone (3)
It was prepared from acenaphthenone (I; 3.36; 0.02 mole) and o-chlorobenzaldehyde (2.81 g; 0.02 mole) exactly in the same manner as described for 2 and usual work up provided 5.1 g (87.7%) of desired compound, m.p. 178-79°. IR (KBr): 3030 (-CH aromatics), 1690 (>C=O), 1620cm-1 (C=C); 1H NMR (CDCl3 + TFA) : 7.96 (1H, s, benzal proton) and 8.20 to 7.20 (10 H, m, rest of aromatic protons).
Photolysis of benzylideneacenaphthenones (2,3) without light in the presence of hydrogen peroxide
A solution of 2 or 3 (0.005 mol) in isopropanol (100 ml) with 2,4-dinitrophenyloxalate (0.006 mol) was stirred at room temperature and 30% hydrogen peroxide (50 ml) was added to reaction mixture dropwise with stirring in the atmosphere of purified nitrogen (1hr). Then the reaction mixture was diluted with water, extracted with chloroform, chloroform extract washed with water and dried (Na2SO4). Evaporation of solvent and column chromatography gave 3.1% of unidentified product, 18% of 10(H) oxo-10, 11 methanochrysene (4 or 6), and 15.5% of naphthalene-1,8-dicarboxylic and anhydride (5). The rest of starting material (2 or 3) was recovered unreacted.
IR (KBr): 3020 (-CH aromatics), 1700 cm-1 (>C=O); mass: 254 (100) (M+)
IR (KBr): 3045 (-CH aromatics), 1770, 1745 cm-1 (C=O, anhydride); 1H NMR (DMSO-d6): d 8.75 (4H, dd, H-2, H-4, H-5 and H-7) and 8.0 (2H, dd, H-3 and H-6); Mass: m/e 198 (59) (M+), 154 (96) (M+–CO2), 126 (100) (M+ – CO2 – CO), 100(22) (M+ – CO2 – CO – C2H2).
IR (KBr): 3060 (-CH aromatic), 1730cm-1 (>C=O); 1H NMR. (DMSO-d6): 8.67 (1H, dd, H-4), 8.37 (1H, dd, H-9), 8.33 (1H, s, H-11), 7.05 to 8.20 (rest of aromatic protons); Mass : m/e 290 (100) and 288(30) (M+)
Acknowledgement
Author is thankful to the Head of Chemistry Department, M.D.University, Rohtak for providing necessary facilities.
References
- White E H. Wiecko, J. & Roswell, D.F., J. Amer. Chem. Soc., 91, 1969, 5194.
- Kopecky K R. & Mumford, C, Can. J. Chem. 92, 1967, 3944.
- White E H, Wieckoand J & Mumford C, Can. J. Chem., 47, 1969, 709.
- Gusten H & Ullman E F, Chem. Commun., 1970, 28.
- White E H & Wildes P D, J. Amer. Chem. Soc., 93, 1971, 6286.
- White E H, Wildes P D, Wiecko J & Wei C C, J. Amer. Chem. Soc., 95, 1973, 7050.
- Othmer K, Encyclopedia of Chemical Technology, IInd Ed. Wiley (Interscience), New York, 15, 1968, 331.
- Fieser F F & James C, J. Amer. Chem. Soc., 62, 1940, 432.
- Nakanishi K, Infrared Absorption Spectroscopy, IInd Ed., Holdix-Day, 1964.
- Jackman I M & Sternhell S, Applications of NMR Spectroscopy in organic chemistry, IInd Ed., Pergamon press, Oxford, 1972.
- Parshad R, Ph.D. Thesis, 1979, 96.

This work is licensed under a Creative Commons Attribution 4.0 International License.









