Spectroscopic and Biocidal Study of Co(Ii), Cu(Ii) and Ni(Ii) Chelates of Nitrogen and Sulphur Containing Schiff Base Derived from 6-Methyl-2-Phenyl-4h-Chromen-4-One
B. K. Rai*, S. N. Vidyarthi1, Puja Sinha2, Vineeta Singh3 and Sanjiv Kumar1
*Department of Chemistry, L. N. T. College, Muzaffarpur, India. B.R.A. Bihar University, Muzaffarpur, India.
1Department of Chemistry, J. P. University, Chapra.
2FNS Academy, +2 Govt. School, Guljarbag, Patna.
3BNR +2 Govt. School, Guljarbag, Patna.
In the present paper, a series of complex of the type [MLX2] where M = Co(II), Ni(II) and Cu(II), L = 6-methyl-2-phenyl-4H-chromen 4-thisemicarbazone (MPCT) have been synthesized. Their structure were confirmed by means of IR, electronic spectra, elemental analysis and molar conductance, coupled with magnetic susceptibility measurements. All the compounds were assayed for antibacterial activities against one gram positive bacterial strain Bacillus subtilis and one gram negative bacterial strain Eschericpia coli, using disc diffusion technique. It was observed on comparison with reference to antibiotic and fungicides. The complexes were found to be more effective than ligands.
KEYWORDS:MPCT; Cobalt(II); Nickel(II); Copper(II); Complexes
Download this article as:| Copy the following to cite this article: Rai B. K, Vidyarthi S. N, Sinha P, Singh V, Kumar S. Spectroscopic and Biocidal Study of Co(Ii), Cu(Ii) and Ni(Ii) Chelates of Nitrogen and Sulphur Containing Schiff Base Derived from 6-Methyl-2-Phenyl-4h-Chromen-4-One. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Rai B. K, Vidyarthi S. N, Sinha P, Singh V, Kumar S. Spectroscopic and Biocidal Study of Co(Ii), Cu(Ii) and Ni(Ii) Chelates of Nitrogen and Sulphur Containing Schiff Base Derived from 6-Methyl-2-Phenyl-4h-Chromen-4-One. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25177 |
Introduction
Schiff bases have the ability to act as coordinating ligands and the polynuclear complexes derived from Schiff bases find variety of applications in analytical and biological systems1-12. Schiff base complexes of transition metals are of great interest and have extensively been studied over past few decades13-16. Keeping in view the importance of Schiff base complexes of transition metal and in continuation of earlier work17-30 on Schiff base coordination compounds, the present work was undertaken to study the complexes of Co(II), Ni(II) and Cu(II) ions with bioactive ligands. In the present paper, we report a series of new Co(II), Ni(II) and Cu(II) complexes of Schiff base 6-methyl-2-phenyl-4H-chromen-4-thiosemicarbazone. The complexes have general molecular formula [MLX2]; where M = Co(II), Ni(II) and Cu(II); L = 6-methyl-2-phenyl-4H-chromen-4-thiosemicarbazone (MPCT) and X = Cl–, Br–, I– and NO3–.
Expermental
All the reagents and solvents were of analytical grade and were obtained from BDH and used as received.
Physical measurements: Analytical data were collected on Perkin Elmer-2400 CHNS/O elemental analyzer. Melting point were taken in open capillary tube and are uncorrected. IR spectra (KBr discs, 4000-200 cm-1) were recorded using Perkin-Elmer-577 spectrophotometer. The molar conductance measurements were carried out at room temperature on Systronics conductivity meter model 303 using DMF as a solvent. The electronic spectra were recorded on Cary 2390 spectrophotometer in the 10000-25000 cm-1 and magnetic susceptibility of the samples were made on a Gouy balance using Hg[Co(NCS)4] as a calibrant.
Preparation of the ligand
The hot ethanolic solution (20 ml) of 6-methyl-2-phenyl-4H-chromen-4 one and hot ethanolic solution of semicarbazide hydrochloride dissolved in sodium acetate was mixed slowly with constant stirring. This mixture was refluxed (80-90oC) for 3-4 h. On cooling, colourless precipitate was formed, which was filtered, washed and dried under vacuum over phosphorus pentaoxide, yield 62%, m.p 180±1oC.
Preparation of the complexes
Hot ethanolic (20 ml) solution of ligand (3.09 g, 0.01 mol) and hot ethanolic solution of corresponding metal salt (0.005 mol) were mixed together with constant stirring. The mixture was refluxed for 2-3 h at 70-90oC. On cooling coloured complex was precipitated out. It was filtered, washed with cold ethanol and dried under vacuum over phosphorus pentaoxide; Yield-58%.
Results and Discussion
I.R. spectra
The I.R. spectrum of the ligand MPCT exhibit strong and broad band at 3160 cm-1 which is assigned31,32 to nN–H. In the spectra of the complexes, this band is unaffected which indicates non participation of either primary amino or secondary amino group in the coordination. IR spectrum of the ligand MPCT exhibits a strong and medium band at 1475 cm-1 assignable31-35 to nC=N. In the spectra of the complexes this band shows
Table 1: Analytical, colour, mol. wt., magnetic susceptibility values, conductivity measurement and decomposition temperature of ligand MPCT and its metal complexes.
|
Compounds (Colour) |
Yield % |
Molar mass |
% Analysis found (calculated) |
DT oC. |
meff B.M
|
Wm ohm-1 cm2 mol-1 |
lmax electronic cm-1 |
|||
|
M |
C |
N |
H |
|||||||
|
MPCT (Colourless) |
68 |
309 |
65,79 (66.01) |
13.42 (13.50) |
4.78 (4.85) |
|||||
|
[Co(MPCT)2Cl2] (Deep brown) |
74 |
747.93 |
7.80 (7.87) |
54.38 (54.55) |
11.14 (11.23) |
3.96 (4.01) |
271 |
4.96 |
4.7 |
9300, 13500, 19700 |
|
[Co(MPCT)2Br2] (Yellow) |
70 |
836.24 |
6.94 (7.04) |
48.64 (48.76) |
9.91 (10.03) |
3.49 (3.56) |
255 |
5.03 |
4.6 |
9200, 13900, 19300 |
|
[Co(MPCT)2I2] (Deep green) |
72 |
930.73 |
6.27 (6.33) |
43.72 (43..83) |
8.94 (9.02) |
3.27 (3.22) |
260 |
5.24 |
5.1 |
9800, 14500, 19800 |
|
[Co(MPCT)2(NO3)2] (Deep green) |
70 |
800.93 |
7.28 (7.35) |
50.83 (50.94) |
10.49 (10.46) |
3.68 (3.74) |
253 |
504 |
5.3 |
9700, 14200, 19500 |
|
[Ni(MPCT)2Cl2] (Yellowish blue) |
74 |
747.71 |
7.69 (7.85) |
54.42 (54.56) |
11.10 (11.23) |
3.97 (4.01) |
232 |
3.13 |
6.1 |
12400, 18300, 25700 |
|
[Ni(MPCT)2Br2] (Yellow) |
71 |
836.57 |
6.94 (7.01) |
48.63 (48.77) |
9.93 (10.04) |
3.50 (3.58) |
243 |
3.16 |
6.7 |
12100, 18400, 24700 |
|
[Ni(MPCT)2I2] (Red) |
73 |
930.51 |
6.22 (6.30) |
43.76 (43.84) |
8.94 (9.02) |
3.15 (3.22) |
238 |
3.18 |
6.4 |
12800, 19000, 25500 |
|
[Ni(MPCT)2(NO3)2] (Green) |
75 |
800.71 |
7.26 (7.33) |
50.78 (50.95) |
10.37 (10.49) |
3.68 (3.74) |
231 |
3.24 |
6.3 |
12700, 18700, 25300 |
|
[Cu(MPCT)2Cl2] (Green) |
70 |
752.54 |
8.37 (8.44) |
54.10 (54.21) |
11.07 (11.16) |
3.92 (3.98) |
217 |
1.87 |
3.1 |
13600, 18300 |
|
[Cu(MPCT)2Br2] (Greenish yellow) |
70 |
841.35 |
7.46 (7.55) |
48.38 (4849) |
9.89 (9.98) |
3.48 (3.56) |
204 |
1.91 |
2.4 |
12700, 17900 |
|
[Cu(MPCT)2(NO3)2] (Green) |
70 |
805.54 |
7.67 (7.88) |
50.49 (50.64) |
10.30 (10.42) |
3.66 (3.72) |
167 |
1.88 |
2.3 |
13100, 18700 |
DT = Decomposition Temperature
Table 2: Salient features of IR spectral bands of ligand MPCT and its metal complexes.
|
Compounds |
nN–H |
nC = N |
nC = S |
nM – O |
nM – N |
nM – S |
nM – X |
|
MPCT |
3160 s,b |
1475 s,m |
800 s,b |
||||
|
[Co(MPCT)2Cl2] |
3160 s,b |
1455 s,m |
770 s,s |
410 m |
395 m |
305 m |
|
|
[Co(MPCT)2Br2] |
3160 s,b |
1450 s,m |
765 s,s |
430 m |
390 m |
250 m |
|
|
[Co(MPCT)2I2] |
3160 s,b |
1445 s,m |
770 s,s |
415 m |
405 m |
270 m |
|
|
[Co(MPCT)2(NO3)2] |
3160 s,b |
1445 s,m |
770 s,s |
505 m |
450 m |
405 m |
|
|
[Ni(MPCT)2Cl2] |
3160 s,b |
1440 s,m |
775 s,s |
445 m |
400 m |
325 m |
|
|
[Ni(MPCT)2Br2] |
3160 s,b |
1440 s,m |
765 s,s |
435 m |
395 m |
295 m |
|
|
[Ni(MPCT)2I2] |
3160 s,b |
1440 s,m |
770 s,s |
445 m |
405 m |
275 m |
|
|
[Ni(MPCT)2(NO3)2] |
3160 s,b |
1445 s,m |
765 s,s |
510 m |
440 m |
390 m |
|
|
[Cu(MPCT)2Cl2] |
3160 s,b |
1440 s,m |
765 s,s |
445 m |
395 m |
315 m |
|
|
[Cu(MPCT)2Br2] |
3160 s,b |
1445 s,m |
770 s,s |
440 m |
395 m |
285 m |
|
|
[Cu(MPCT)2(NO3)2] |
3160 s,b |
1445 s,m |
770 s,s |
500 |
445 m |
390 m |
m = medium, s = strong, b = broad
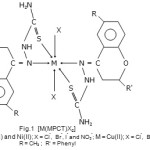 |
Scheme 1 Click here to View scheme |
Red shift with slightly reduced intensity. The shift of the band and change in intensity proposes coordination of the azomethine N with metal ion. The infrared spectrum of the ligand shows a strong and broad band at 800 cm-1 assigned31,32,34 to nC=S. In the spectra of the complexes this band also shows red shift indicating coordination takes place through thione S atom of thiosemicarbazone moiety.
The conclusive evidence of bonding of ligand to metal ion through oxygen atom of either nitrate or perchlorate ion, N atom of azomethane group and thione S of thiosemicarbazone moiety is supported by the appearance of bands31,32,35 due to, nM-O at 500-510 cm-1, nM-S at 390-405 cm-1, nM-N at 435-450 cm-1 respectively. The evidence of metal-halogen linkage is supported by the low molar conductance values35 of the complexes in the range 2.3-6.7 ohm-1 cm2 mol-1 and appearance of a band in the region 315-275 cm-1 which assigned31,32,35 to nM-X.
Nitrate complexes show characteristic medium intensity bands at 1300 and 1140 cm-1 with a separation of 140 cm‑1 due to monodentate coordinated nitrate37 group.
On the basis of above discussion on IR spectral data, it is proposed that the ligand MPCT acts in a neutral bidentate manner. The remaining coordination positions of metal ions are satisfied by negative ions, such as Cl–, Br–, I– or NO3–.
Biocidal study
Schiff base MPCT and their metal complexes have been evaluated for their antimicrobial activity against E. coli and B. subtilis by disc diffusion method41 at concentrations 50 and 25 mg ml-1 using streptomycin as control. On comparison with reference to antibiotic, the complexes were found to be more effective than free ligand. Further, it is also observed that the order of activity for complexes are: Cu(II)>Ni(II)>Co(II) and also nitrate complexes have effective biocidal effect than metal-halide complexes which is supported by literature42-44.
Electronic spectra and magnetic susceptibility of the complexes
The electronic38 spectral and magnetic39,40 susceptibility values tentatively proposes octahedral geometry of Co(II), Ni(II) and Cu(II) complexes.
Acknowledgement
The author is grateful to the U.G.C. for providing financial assistance under Minor Research Project.
References
- Ghiladi M., Larsen F. B., Mckenzie C. J., Sotofte I. and Tuchogues J. P., J. Chem Soc., Dalton Trans, 1687 (2005).
- Chakraborty J., Nandi M., Mayer Figge G., Sheldrick W. S., Sorace L., Bhaumik A. and Banerjee P., Eur. J. Inorg. Chem., 5033 (2007).
- Eryleben A. and Hermann J., J. Chem. Soc., Dalton Trans., 569 (2000).
- Biswas P., Ghosh M., Dutta S. K., Florke U. and Nag K., Inorg. Chem., 45, 4830 (2006).
- Mishra L. and Sinha R., Indian J. Chem., Sect.A, 29, 1131 (2000).
- Mukhopadhyay and Rau D., Indian J. Chem., Sect.A, 40, 228 (2001).
- Krishnankutty K. and Ummathur M. B., J. Indian Chem. Soc., 83, 633 (2006).
- Dubey R.K., J. Indian Chem. Soc., 83, 1087 (2006).
- Saritha P., Reddy B. S. and Jayatyagaraju, J. Indian Chem. Soc., 83, 1204 (2006).
- Gulloli M., Casella L., Pasini A. and Ugo R., J. Chem. Soc., Dalton Trans., 339 (1977).
- Muslim L., Roth W. and Erienmeyor H.H., Acta Chem. Helv., 36, 36 (1983).
- Vyas R. R. and Mehata R.N., J. Indian hem. Soc., 68, 294 (1991).
- Gruber S.J., Harris C.M. and Sinn E., Inorg. Chem., 7, 268 (1968).
- Gruber S. J., Harris C. M. and Sinn E., J. Inorg. Nuclei Chem., 30, 1805 (1968).
- Gruber S. J., Harris C. M. and Sinn E., J. Inorg. Nuclei Chem., 18, 3469 (1979).
- Fachinetti G., Floriani C., Zanzzi P.F.,and A. R. Zanzari, Inorg. Chem., 18, 3469 (1979).
- Rai B. K., Asian J. Phys., 16, 71 (2007); Rai B. K., Rai H. C., Singh Shiv Pujan, Tomar Rashmi, Prakash Om and Sahi Poonam, Asian J. Phys., 26, 76 (2007).
- Rai B. K. and Sharma K., Asian J. Chem., 20, 137 (2008).
- Rai B. K., Rai Rajeshwar, Sahi Poonam and Rana Swaty, Asian J. Chem., 30, 143, 149 (2008).
- Rai B. K., Hussain Z., Singh U. P., Prasad S. N., Prasad A and Mishra P.M., J. Ultra Chem., 4, 53 (2008); Rai B. K. and Prasad S. N., J. Ultra Chem, 4, 71 (2008); Rai B. K. and Kumar A., J. Ultra Chem., 4, 179 (2008).
- Rai B. K., J. Ind. Council Chem., 25, 137 (2008); Rai B. K., Baluni A., Prasad A., Thakur R. and Prakash P., Asian J. Chem, 21, 3708, 3713 (2009).
- Rai B. K. and Vinayak, J. Ultra Chem, 5, 67 (2009); Rai B. K., Kumar A., Ravishankar, J. Ultra Chem, 5, 73 (2009); Rai B. K., Kumari S., Singh R. K., Prasad A., Sinha M. Prasad and Mishra P. M., J. Ultra Chem, 5, 83 (2009).
- Prasad A.and Rai B. K., Orient J. Chem., 25, 175 (2009); Rai B. K., Ravishankar and Pandey S., Asian J. Chem., 21, 5409 (2009).
- Rai B. K., Prasad A., Vinayak, Singh S. P. and Jha S. ‘Sunit’, Asian J. Phys., 18, 63 (2009); Rai B. K., Singh Vineeta, Vinayak, Singh S. P. and Jha S. ‘Sunit’, Asian J. Phys., 6, 18, 67 (2009).
- Rai B. K., Kumar H., Sharma M. and Rastogi V. K., J. Indian Chem Soc., 87, 1241 (2010).
- Rai B. K., Asian J. Chem., 22, 2761 (2010); Rai B. K. and Kumar Chandan, Asian J. Chem., 22, 5613 (2010); Rai B. K. and Singh Sateydeo, Asian J. Chem., 22, 5619 (2010); Rai B. K. and Sharma K. K., Asian J. Chem., 22, 5625 (2010); Rai B. K., J. ind. Council Chem., 27, 68 (2010).
- Kishore K. R. and Rai B. K., Asian J. Chem,, 22, 8055 (2010); Rai B. K. and Kumar Bimal, Asian J. Chem., 22, 8073 (2010); Rai B. K. and Singh S., Orient J. Chem., 26, 989 (2010); Rai B. K. and Kumar Chandan, Orient J. Chem., 26, 1019 (2010); Rai B. K. and Kumar Bimal, Orient J. Chem., 26, 1097 (2010).
- Rai B. K., Sinha Puja, Vidyarthi S. N. and Singh Vineeta, Asian J. Chem., 23, 4629 (2011); Rai B. K. and Kumar Bimal, Asian J. Chem., 23, 4635 (2011); Rai B. K., Singh Vineeta, Vidyarthi S. N. and Sinha Puja, Asian J. Chem., 23, 4638 (2011).
- Rai B. K.l and Anand Puja, Orient J. Chem; 28, 525 (2012); Rai B. K., Kumari Rachana and Thakur Amrita, Orient J. Chem., 28, 943 (2012); Rai B. K., Vidyarthi S. N., Sinha Puja, Singh Kalyan Chandra, Sahi Shashi Bhushan and Jha Javvir, Sharan, Orient J. Chem., 28, 1365 (2012); Rai B. K., Vidyarthi S. N., Amit Singh Rabindra, Bhardwaj Nithish and Ojha Avinash, Orient J. Chem., 28, 1403 (2012).
- Rai B. K. aand Anand Rahul, Asian J. Chem., 25, 480 (2013); Rai B. K., Thakur Amrita and Divya, Asian J. Chem., 25, 583 (2013). Rai B. K., Vidyarthi S. N., Kumari Punam, Kumari Suman, Kumari Laxmi and Singh Rajkishore, Asian J. Chem., 25, 941 (2013); Rai B. K. and Kumar Arun, Asian J. Chem., 25, 1169 (2013).
- Silverstein Robert and Webster X. Francis, “Spectrometric Identification of Organic Compounds”, 6th ed., John Wiley & Sons (2008).
- Kemp William, “Organic Spectroscopy”, 3rd ed., Palgrove, New York (2008).
- Gudasi K. B., Patil S. A., Vadavi R. S., Shenoy R. V. and Patil R. S., J. Serb. Chem. Soc., 17, 526 (2006).
- Agarwal R. K., Agarwal H. and Chakraborti I., Synth. React. Inorg. Metal Org. Chem., 25, 679 (1995).
- Ferraro J. R., “Low Frequency Vibration of Inorganic and Coordination Compound”, Plenum Press, New York.
- Boghaei D. M. and Zadegan N. Lashani, Synth. React. Inorg. Metal-Org. Chem, 30, 1393 (2000).
- Addition C. C., Logan N., Wallwork S. C. and Barmer D. C., Quart. Rev. (1971).
- Krishna C. H., Mahapatra C. M.l and Dash A. K., J. Inorg. Nucl. Chem., 39, 1253 (1977).
- B. N. Figgis, “Introduction to Ligand Field”, Wiley Eastern Ltd., New Delhi, 279 (1976).
- Carlin R. L. and Dryneveledt A. J. Van, “Magnetic Properties of Transition Metal CCompounds”, Springer Verlag, New York, (1997).
- Mukherjee P. K., Saha K., Giri S. N., Pal M. and Saha B. P. , Indian J. Microbiology, 35 (1995).
- Parashar R. K. and Sharma R. C., J. Inorg. Biochem, 28, 225 (1987).
- Rainsford K. D. and Whitehouse M. W., J. Pharmacol, 28, 83 (1976).
- Nishant N., Ahmad S. and Ahmad R. T., J. Appl. Polym. Sci., 100, 928 (2006).

This work is licensed under a Creative Commons Attribution 4.0 International License.









