Racemisation of (1R, 6S)- 8-Benzyl-7,9-Dioxo-2,8-Diazabicyclo[4.3.0]Nonane using Halogenated Solvent in Basic Medium
Jigar Bhavsar1, Bhuwan Bhashkar1 and Anil Kumar2*
1Chemical Research Department 191-E, Sector 4-II, IMT-Manesar, Haryana - 122 050, India.
2Mankind Research Centre, Sector 4-II, IMT-Manesar, Haryana - 122 050, India.
A simple, highly rapid, efficient and eco friendly process for Racemisation of (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0] nonane in various halogenated solvents has been carried out as a base catalysed reaction to afford racemic mixture in excellent yields (80-95%) at temperature of or below 40oC. The base catalysed racemisation in halogenated solvent has an advantage of simple workup procedure, and use of less volume of halogenated solvent irrespective of type of solvent used in the reaction system.
KEYWORDS:Racemisation; Undesired isomer; Halogenated solvent; Moxifloxacin
Download this article as:| Copy the following to cite this article: Bhavsar J, Bhashkar B, Kumar A. Racemisation of (1R, 6S)- 8-Benzyl-7,9-Dioxo-2,8-Diazabicyclo[4.3.0]Nonane using Halogenated Solvent in Basic Medium. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Bhavsar J, Bhashkar B, Kumar A. Racemisation of (1R, 6S)- 8-Benzyl-7,9-Dioxo-2,8-Diazabicyclo[4.3.0]Nonane using Halogenated Solvent in Basic Medium. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25158 |
Introduction
Fluoroquinolones are well tolerated drugs and amongst them the fourth generation fluoroquinolones act at DNA gyrase and topoisomerase II and IV1. One of the widely used fourth generation fluoroquinolone is moxifloxacin (Figure 1). It has enhanced activity against specific bacteria, such as Mycobacteria spp. and Legionella. Moxifloxacin designed to treat community-acquired respiratory tract infections, showed better efficacy than ciprofloxacin against S. maltophilia.
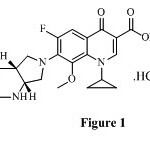 |
Figure 1 Click here to View figure |
For preparing the quinolone derivative like moxifloxacin, there is required enantiomerically pure (S,S)-2,8-diazabicyclo[4.3.0] nonane (Figure 2), also referred as cis-S,S-pyrrolopiperidine which is known in the literature3-8 as in scheme 1.
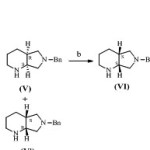 |
Scheme 1 Click here to View scheme |
There are few methods known from the literature9, where resolution was performed on compound (IV) as shown in scheme 2. It is advantageous to resolve a compound into single enantiomer during the initial stages of the process, as reduction of diones is performed on chiral compound thereby requiring less amount of reducing agent as will be required when performed on racemic compound, to obtain high throughput in the synthesis.
As enantiomerically pure (1S,6R)-8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane is required for synthesis of (S,S)- 2,8-diazabicyclo[4.3.0]nonane , an important intermediate for preparation of moxifloxacin, the resulting undesired enantiomer, (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane (V’) obtained after resolution of compound (III), cannot be used any further. This has the disadvantages that the undesired enantiomer, which is useful by itself, is lost and that its disposal involves costs.
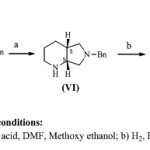 |
Scheme 2 Click here to View scheme |
A number of routes for racemizing (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane (V’) and recycling of said undesired enantimer is known from literature. One of the method10 is shown in scheme 3. Racemisation of undesired isomer was performed using manganese dioxide which led to the elimination of two stereocenters resulting into formation of tetrahydropyridine derivative, and the dehydrogenated compound thus obtained was then rehydrogenated to give racemate. It is observed that the use of manganese dioxide results into formation of equivalent amount of manganese oxide as a side product which makes reaction mass thick and hence need to be separated periodically from the reaction mass. Another approach11 for racemisation of undesired enantiomer (V’) is shown in scheme 4 and involves N-chlorination of (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane (V’) using sodium dichloroisocyanurate, and was dehydrogenated using base like triethyl amine. Before it is possible to recycle the undesired enantiomer to racemate, all dehydrogenations require the hydrogenation of the resulting compounds as an additional step. Taken as a whole, racemisation by dehydrogenation is either unsuitable in principle for industrial application or, requires ultimate safety precautions for hydrogenations and is technically multifaceted and unproductive.
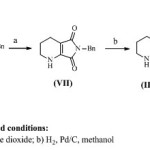 |
Scheme 3 Click here to View scheme |
Another racemisation process reported in literature12 involved substoichiometric amount of alkoxide as base in solvents like tetrahydrofuran and toluene. It elucidated that depending on the choice of the solvent, it is possible to carry out the racemisation in relatively highly concentrated reaction mixture. For example, when solvent used is non-aromatic aprotic solvent, concentration of (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane (V’) in the solvent can be up to 50% by weight whereas in case of aprotic aromatic solvents, concentration of (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane (V’) can be up to 1/4th of the solvent.
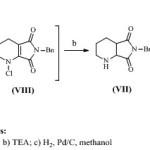 |
Scheme 4 Click here to View scheme |
We have developed an efficient racemisation process as in scheme 5, using less amount of base in halogenated solvent wherein, halogenated solvent can be aromatic or non-aromatic halogenated solvent. The racemisation process has an advantage that 1) it goes to completion with ease and without much effort, 2) does not require expensive solvents like tetrahydrofuran, 3) evading side product formation, 4) ease of work up and purification processes, 4) no extra step of hydrogenation is required and most importantly 5) the concentration of undesired enantiomer (V’) in the halogenated solvent can be taken up to 50% irrespective of choice of halogenated solvent i.e. halogenated solvent employed for racemisation process can be taken in single or mixture of more than one and can be aromatic or non-aromatic solvent. In the present work, eco-friendly method is employed for racemisation of undesired enantiomer which can be conveniently scaled up to an industrial process of synthesis of moxifloxacin.
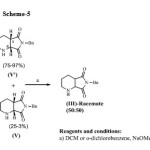 |
Scheme 5 Click here to View scheme |
Experimental
1H NMR spectra were recorded in Bruker 500-MHz spectrometer using TMS as internal standard (chemical shifts in δ, ppm). The mass analysis was performed on Waters Xevo TQD LC-MS/MS mass spectrometer. Solvent removal was accomplished by rotary evaporator operating at vacuum of 40-50 Torr. All the reactions and homogeneity of products formed were monitored by chiral HPLC.
General Procedure
To 250ml of methylene dichloride was added 100g of undesired compound V’ (chiral purity: 97%) followed by slow addition of 7.11g (0.0395mol) of sodium methoxide at room temperature and stirred the reaction mass under inert atmosphere for 1h. Progress of reaction was monitored by chiral HPLC. After completion of reaction, added 2.37g (0.0395mol) of acetic acid at room temperature and stirred the resulting solution for 30 min. Added 50ml of de-mineralized water and separated the layers. Organic layer was washed with 50ml of water and dried over anhydrous sodium sulphate. Filtered and concentrated the organic layer under reduced pressure to get 94g of racemic compound (49.8: 50.2 by chiral HPLC). 1H NMR (500MHz, MeOD): δ 7.12-7.21 (m, 5H), 4.51 (s, 2H), 3.79-3.81 (d, 1H), 3.20 (s, 1H), 2.75-2.79 (q, 1H), 2.61-2.66 (m, 1H), 2.43-2.47 (m, 1H), 1.81-1.86 (1H, m), 1.44-1.50 (m, 1H), 1.31-1.38 (m, 2H) ; MS: 245 (M+1, 100%).
Table 1: Racemisation of (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane of formula (V’) using different halogenated solvents and varied mole equivalents of base.
|
S.No |
Undesired isomer (chiral purity) |
Base |
Mole equivalent |
solvent |
Ratio of V’ to solvent |
Yields [racemate (49.8:50.2)] |
|
1 |
V’ (97%) |
NaOEt |
0.1 |
Methylene dichloride |
1:1 |
90% |
|
2 |
V’ (97%) |
NaOEt |
1.0 |
Methylene dichloride |
1:1.5 |
72.5% |
|
3 |
V’ (97%) |
NaOEt |
0.5 |
Methylene dichloride |
1:1.5 |
79% |
|
4 |
V’ (97%) |
KOt-Bu |
0.09 |
Dichloroethane |
1:1 |
93% |
|
5 |
V’ (73%) |
KOt-Bu |
0.5 |
Methylene dichloride |
1:2 |
82% |
|
6 |
V’ (75%) |
NaOMe |
1.0 |
o-dichlorobenzene |
1:1 |
75% |
|
7 |
V’ (97%) |
NaOMe |
0.08 |
o-dichlorobenzene |
1:1 |
92.5% |
|
8 |
V’ (97%) |
KOt-Bu |
1.0 |
Methylene dichloride |
1:2 |
74.6% |
|
9 |
V’ (75%) |
NaOMe |
0.5 |
Dichloroethane |
1:2 |
83% |
|
10 |
V’ (73%) |
NaOEt |
0.1 |
Dichloroethane |
1:1.5 |
92.2% |
|
11 |
V’ (97%) |
NaOEt |
0.5 |
o-dichlorobenzene |
1:1 |
80% |
Experiment 12
To the solution of 165g (0.416mol) of (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane mono tartarate salt in 250 ml of methylene dichloride was added 250 ml of water at 25oC. Adjusted the pH of the reaction mass to 9.0-9.5 by addition of aq. sodium hydroxide solution and separated the two layers. To the organic layer containing the free base of (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane, was then added 7.11g (0.0395mol) of sodium methoxide at room temperature and stirred the reaction mass under inert atmosphere for 1h. Progress of reaction was monitored by chiral HPLC. After completion of reaction, added 2.37g (0.0395mol) of acetic acid at room temperature and stirred the resulting solution for 30 min. Added 50ml of de-mineralized water and separated the layers. Organic layer was washed with 50ml of water and dried over anhydrous sodium sulphate. Filtered and concentrated the organic layer under reduced pressure to get 92g of racemic compound (49.8: 50.2 by chiral HPLC).
Result and Discussion
The present work was focused on development of eco-friendly process for racemisation of undesired enantiomer, (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane (V’). The racemisation of undesired isomer was carried out in halogenated solvents using base with different mole equivalents. The initial studies were focused on the optimisation of the reaction conditions for the racemisation of (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane (V’). The model reaction was carried out by taking stoichiometric amount of base and compound (V’) having chiral purity of 97% in halogenated solvent like methylene dichloride affording the desired racemate compound in 80% yield. Firstly, Effect of mole equivalents of base were studied to optimize the reaction conditions and it was observed that as the mole equivalents of base are decreased there is an improvement in the yield of the racemate compound.. From this study, it was seen that even in 0.08 mole equivalents of base, considerable yields (above 90%) were achieved.
Secondly, effect of the type of halogenated solvent as summarized in Table 1, was studied and it was observed (as shown in experiment no. 3,5,9 and 11) that there was no major variation in the yield of the desired racemate in respect to whether the solvent used is aromatic or non aromatic halogenated solvent. The major observation was that the racemisation can be carried out in relatively highly concentrated reaction mixture where the concentration of undesired isomer, (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane (V’) can be up to 50% by weight of solvent irrespective of the type of solvent used in the reaction system.
In order to minimise the undesirable side reactions, it is advantageous to carry out the racemisation under substantial exclusion of oxygen and to carry out the racemisation under inert atmosphere. The racemisation according to the invention can be carried out at temperature of or below 40oC.
As per experiment no. 12, a plausible method of preparation of racemic compound from undesired enantiomer, (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane (V’) has been proposed wherein, compound (V’) is not isolated after salt hydrolysis and is in situ proceeded for racemisation process. By performing reaction according to experiment 12, one can skip processes like distillation of solvent and drying of compound (V’).
Conclusion
In conclusion, we have found a method, based on two major quantitative i.e. catalytic amount of base and less volume of halogenated solvent, irrespective of type of solvent, for racemisation of undesired isomer, (1R, 6S)- 8-benzyl-7,9-dioxo-2,8-diazabicyclo[4.3.0]nonane (V’) so as to make it recyclable into the resolution process. The racemisation according to the invention has the advantage that it is technically simple, avoid steps like dehydrogenation followed by rehydrogenation and involves inexpensive reactions. This procedure is of great importance for moxifloxacin manufacture and, susceptible to many advantageous applications with other substrates.
Acknowledgement
We thanks analytical group of Advanced Instrumentation Research Facility, Jawaharlal Nehru University for their generous support for providing 1H NMR 500MHz analysis.
References
- Drlica, K. and Zhao, X., Microbiol Mol Biol Rev., 61 (3), 377–92 (1997).
- Denton, M. and Kerr, K., Clin Microbiol Rev., 11 (1), 57–80 (1998).
- Ume, P.; Thomas, S.; Andreas, K.; Klaus, K.; Michael, S.; Ingo, H.; Karl, G.M.; Rainer, E. and Hans-Joachim, Z., US4990517 (1991)
- Herbett, D.; Andreas, K.; Elvira, K.; Walter, L.; Hanns-Ingolf, P.; Dietrich, S.; Rolf, G. and Tobias, R., US2007/0213526 A1 (2007)
- Radharkrishna, T.; Sreenivas Rao, D.; Vyas, K. and Reddy, G., Journal of Pharmaceutical and Biomedical Analysis, 22(4), 691 (2000)
- Xiongfei, T., CN101591336 (2009)
- Uwe, P.; Thomas, H.; Thomas, S.; Andreas, K.; Klaus, G.; Dieter, B.K.; Karl, G.M.; Rainer, E. and Hans-Joachim, Z., US5468742 (1995)
- Uwe, P.; Wilfred, S.; Dieter, H.; Andreas, K.; Thomas, S.; Thomas, P.; Klaus, G.; Rainer, E.; Klaus-Dieter, B. and Karl-Georg, M., US5480879 (1996)
- Yasuhisa, K. and Osamu, U., JP2001039979 (2001)
- Zou, X.; Cai, H. and Zheng, Z., CN101429199 (2009)
- Pallavicini, M.; Bolchi, C.; Fumagalli, L.; Piccolo, O. and Valoti, E., Tetrahedron: Asymmetry, 22(4), 379-380, 2011
- Herbett, D.; Georg, M.; Wilfried, J.; Andreas, K. and Joachim, W., US6392044 B1 (2002)

This work is licensed under a Creative Commons Attribution 4.0 International License.









