Preparation of Calcium ion Selective Electrode using Calcium Lactate Enzyme as an Electro Active Material
A. Vijayalakshmi and J. Thamarai Selvi
Department of Chemistry, Avinashilingam Institute for Home Science and Higher Education for Women, Coimbatore - 641 043, India.
A biosensor based on Calcium lactate enzyme was developed for the determination of calcium ions on the activity of the enzyme. The biosensor had a linear relation to calcium concentration 1M to 1x10-4M and used without an internal solution to give a near–Nernstian responses in with detection limits of the order of 10-4M. The stable potentiometric signals are obtained within a short time period of 1 minute. The effect of pH has been studied to found a better response range. The potentiometric selectivity coefficient values were evaluated. The sensors have also been used for the determination of concentration of Ca2+ in milk.
KEYWORDS:Calcium ion selective electrode; Glutaraldehyde (GA); Potentiometer; Cross linking; Immobilization
Download this article as:| Copy the following to cite this article: Vijayalakshmi A, Selvi J. T. Preparation of Calcium ion Selective Electrode using Calcium Lactate Enzyme as an Electro Active Material. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Vijayalakshmi A, Selvi J. T. Preparation of Calcium ion Selective Electrode using Calcium Lactate Enzyme as an Electro Active Material. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25188 |
Introduction
Calcium and calcium salts are very important minerals used in food industry. Different calcium salts have been studied for decay prevention, sanitation and nutritional enrichment of fresh fruits and vegetables. Calcium lactate, calcium propionate and calcium gluconate have shown some of the benefits of the use of calcium chloride such as product firmness improvement1.
Nowadays, Potentiometry at a trace level is a routine technique. Ion-selective sensors with improved detection limits are investigated; these results in recent dramatic improvements of sensor selectivity and lower detection limit2-8. Electro chemical enzyme based transducers are attractive due to their ease in detection of target analytes, high sensitivity, low cost, simplicity9.
In this study, a new biosensor was developed based on the Calcium lactate enzyme by linking via glutaraldehyde.
Experimental Part
Materials and Methods
Glutaraldehyde(Merck), Disodium hydrogen phosphate, Potassium dihydrogen phosphate, Sodium chloride, Calcium carbonate all are obtained by analytical grade. The standard stock solutions (1M CaCl2) were prepared using distilled water; working solutions were made by dilution of the stock solution.
A digital potentiometer (EQUIP – TRONICS EQ 602) with Ag /AgCl electrode as a reference electrode were used for this study.
Preparation of enzyme
To a dilute solution of cane sugar, a little of sour milk is added. Temperature is maintained at 40 – 450C for six days. The bacillus acidi lacti brings forth fermentation. Methyl glyoxal forms as intermediate compound. Acid is removed by the addition of CaCO3which precipitates calcium lactate.
Immobilization of copper wire with calcium lactate
In this part, we developed calcium electrode based copper wire by immobilizing with calcium lactate and cross linking molecule (Glutaraldehyde). We prepared 2.5 % GA solution in 0.1 mM phosphate buffer solution (PBS) as well as calcium lactate solution in PBS having a concentration of 2 mg/ ml of enzyme. After mixing GA and enzyme solution in one bottle, we dipped the copper wire into it into the solution of enzyme for 15 min to ensure the surface of the copper wire with the monolayer of enzyme. We found that all time gaps show the same result. The immobilized copper wire was taken as working electrode and silver–silver chloride electrode as a reference electrode and the wire was conditioned for 48 hours to attain equilibrium in 1M CaCl2 solution. The EMF measurements were carried out using the following cell assembly,

Results and Discussion
The electrode was first conditioned in 1M CaCl2 solution to attain stable equilibrium .In order to test the performance of the electrode; various parameters including response time, selectivity, and the influence of pH were investigated. The electrode was used over a period of 3 months with good reproducibility .The electrode was kept in deionized water throughout the study.
Response characteristics of the electrode
The electrode potential for a series of standard solution of Ca (II) ions was measured. The electrode gave a linear response to Ca (II) concentration in the range of 1M to 1x 10-4 M. The values are given on table 1.
Table 1: Electrode response for Ca2+ ions
|
S.NO |
Concentration of CaCl2 solution (M) |
EMF (volts) |
|
1 2 3 4 5
|
1 1×10-1 1×10-2 1×10-3 1×10-4
|
0.201 0.160 0.127 0.091 0.051
|
Standard Electrode potential (E0) determined by extrapolation method was found to be +202 V. The slope value was found to be 36 mv/decade. This shows that the electrode behaves according to Nernst equation.
Electrode Response
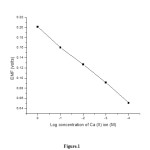 |
Figure 1: Plot of log concentration of Ca(II) ion Vs EMF in volts |
Effect of pH
To study the effect of pH, a standard solution containing 1M CaCl2 ion were prepared in which a series of buffer solution was added. As it can be seen, the potential response remains almost constant over the pH range 4.63- 6.24, which can be taken as the working pH range of the electrode. The decreasing potential at lower pH means that the electrode responses to hydrogen ions.
Selectivity
The selectivity which is an important characteristic of a membrane sensor is measured in terms of the potentiometric selectivity coefficient.
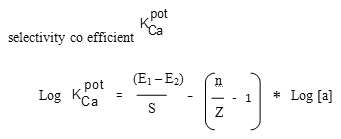
The reduced form of Eisenman’s equation10 given below is often used to calculate the selectivity co efficient
Where E1 and E2 are the potentials measured in 1 M solutions of interfering ion and Ca2+; S is the calibration slope, n and z are the charges of Ca2+ and interfering ions respectively and [a] is the concentration of the ions used. In this method, the concentration of primary ion,Ca(II) ion is varied where as the concentration of secondary interfering ions is kept constant in the test solution which is 1×10-1 M, concentration of interfering ion present case. It was found that the potential remains unaffected in the presence of a series of various cation like Na+ ,Mg2+, Cu2+, K+,NH4+and anion like I–, Br–, Cl–.
Analytical Applications
To assess the applicability of the sensors to real samples an attempt was made to determine calcium ion in real samples like milk, water samples. The recovery of calcium ion in sample analysis was formed to be quantitative with the maximum recovery of 97%.
Refernces
- S.M. Alzamora, D. Salvatori, M.S. Tapia, A. Lopez-Malo, J. Welti-Chanes, P. Fito “Novel functional foods from vegetable matrices impregnated with biologically active compounds” Journal of Food Engineering, (2005), (67),205–214.
- T. Sokalski, A. Ceresa, T. Zwickl, E. Pretsch, J. Am. Chem. Soc. (1997) ,(1191),1347.
- T. Sokalski, A. Ceresa, M. Fibbioli, T. Zwickl, E. Bakker, E. Pretsch, Anal. Chem. (1999) ,(71),1210.
- A. Ceresa, E. Bakker, D. Gunther, B. Hattendorf, E. Pretsch, Anal. Chem. 73 (2001) 343.
- A. Ceresa, A. Radu, S. Peper, E. Pretsch, Anal. Chem. (2002), (74), 4027.
- T. Sokalski, I. Bedlechowicz, M. Maj-Zurawska, A. Hulanicki, Fresenius J. Anal. Chem. (2001) (370): 367.
- A. Ceresa, T. Sokalski, E. Pretsch, J. Electroanal. Chem. (2001),(501): 70.
- A. Malon, R. Radu, W. Qin, A. Ceresa, M. Maj-Zurawska, E. Bakker,E. Pretsch, Anal. Chem. 2003), (75): 3865
- Luo, X; Morrin, A.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis (2006) (18), 319- 326.
- Hassan S S M, Ali M M & Attawiya Amr M Y, Talanta, (2001), (54): 1153.
- Laksh.R.L; “Electroanalytical techniques”, ( 2003) 209-214.
- Bakker E,Buhlmann P&Prestsch E, chem. Rev, (1997),(97),30383;
- Buhlmann.P,PretschE &Bakker, E,chem. Rev, (1998).98:15993
- Zafar Hussain Ibupoto , Syed Muhammad Usman Ali Shah, Kimleang Khun and Magnus Willander, Sensors (2012), (12)2456-2466.

This work is licensed under a Creative Commons Attribution 4.0 International License.









