Mer-R,S-[Znl2](NO3)2, New Zinc Complex With N-(2-Aminoethyl)-1,3-Propanediamine: Spectral and Structural Study
Mohammad Hakimi*, Zahra Mardani and Keyvan Moeini
Chemistry Department, Payame Noor University, 19395-4697 Tehran, I. R. Iran.
In this work, a new zinc complex of N-(2-aminoethyl)-1,3-propanediamine (L), mer-R,S-[ZnL2](NO3)2 (1), was prepared and identified by elemental analysis, FT-IR and Raman spectroscopy and single-crystal X-Ray diffraction. The zinc atom in 1 which is coordinated by four nitrogen atoms of the NH2 groups and two nitrogen atoms of the NH groups has a distorted octahedral geometry. If the distortion in the [ZnL2]2+ cation is disregarded, it has a C2axis and C2symmetry. The dihedral angle between two planes of the coordinated atoms of each ligand confirms the mer conformation. The N–H···O hydrogen bonds between the nitrate and amine groups are presented in the crystal network of 1.
KEYWORDS:Zinc Complex; mer Conformation; X-ray Crystal Structure; C2 Symmetry
Download this article as:| Copy the following to cite this article: Hakimi M, Mardani Z, Moeini K. Mer-R,S-[Znl2](NO3)2, New Zinc Complex With N-(2-Aminoethyl)-1,3-Propanediamine: Spectral and Structural Study. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Hakimi M, Mardani Z, Moeini K. Mer-R,S-[Znl2](NO3)2, New Zinc Complex With N-(2-Aminoethyl)-1,3-Propanediamine: Spectral and Structural Study. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25141 |
Introduction
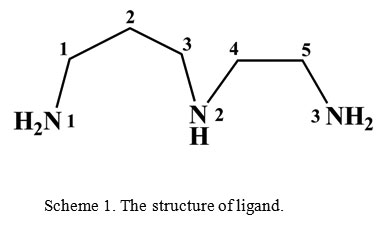
N-(2-Aminoethyl)-1,3-propanediamine (aepn) is a rather common tridentate amine ligand. It usually forms three coordination bonds with the same central atom1 and only rarely aepn adopts bridging function2. In the past few years we have studied complexation of multi N-donor ligands3−6. In continuation of our previous studies, in this work the preparation of new zinc(II) complex, mer–R,S-[ZnL2](NO3)2 (1), with N-(2-aminoethyl)-1,3-propanediamine (L, scheme 1) was described. This complex was characterized by elemental analysis, FT-IR and Raman spectroscopy and X-ray crystallography.
Material and Methods
General Methods
All starting chemicals and solvents were reagent or analytical grade and used as received. Raman spectra were obtained using a Nicolet Model 910 Fourier-transform spectrometer. The infrared spectrum of a KBr pellet was recorded in the range 4000–400 cm−1 using a FT-IR 8400-Shimadzu spectrometer. The carbon, hydrogen and nitrogen contents were determined in a Thermo Finnigan Flash Elemental Analyzer 1112 EA. Melting point was determined using a Barnsted Electrothermal 9200 electrically heated apparatus.
Synthesis of mer–R,S-[ZnL2](NO3)2, 1.
Zn(NO3)2∙6H2O(1 mmol, 0.30 g ) was dissolved in EtOH (15 mL) and added with stirring to a solution of L(2 mmol, 0.23 g) in EtOH (5 mL). A colorless precipitate was formed and after 3 h was filtered and recrystallized from 1:1 H2O/EtOH. Colorless crystals suitable for X-ray diffraction were obtained after 6 days. Yield (0.35 g) 82%; m.p.: 219 °C. Anal. Calcd for C10H30N8O6Zn(%): C, 28.34; H, 7.14; N, 26.44. Found: C, 28.12; H, 7.11; N, 26.58. IR (KBr, cm−1): 3313 s (νas NH2), 3263 s (νs NH2), 3163 s (ν NH), 2916 and 2870 m (ν CH2), 1597 m (δ NH2), 1381 vs (ν4 NO3), 1291 m (ν1 NO3), 1157 m (ν CN), 1088 m (ν2 NO3), 833 w (ν6 NO3), 625 w (ν ZnN). Raman (cm−1): 2904 m and 2810 w (ν CH2), 1534 m (δ NH2), 1420 s (ν4 NO3), 1235 s (ν1 NO3), 1079 s (ν2 NO3), 600 m (ν ZnN).
Crystal structure determination and refinement
Suitable crystals of 1 were placed on an Oxford Diffraction Gemini Ultra diffractometer, and kept at 150.0 K during data collection. Using OLEX-II7, the structures were solved with the SHELXS8 structure solution program using Direct Methods and refined with the SHELXL8 refinement package using least squares minimization. Table 1 contains crystallographic data and details of the data collection and structure refinement. Selected bond lengths (Å) and angles (°) and dimensions of the hydrogen bonds (Å and °) for complex are listed in table 2 and table 3, respectively.
Table 1: Crystal data and structure refinement for 1.
| Empirical formula | C10H30N8O6Zn |
| Formula weight (g mol−1) | 423.79 |
| Temperature (K) | 150.0 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| Unit cell dimensions (Å, °) | |
| a | 11.3391(3) |
| b | 12.3777(3) |
| c | 13.4039(4) |
| β | 90.284(3) |
| Volume (Å3), Z | 1881.24(9), 4 |
| Calculated density (g cm−3) | 1.496 |
| Absorption coefficient (mm−1) | 1.349 |
| F(000) | 896.0 |
| Crystal size (mm3) | 0.09 × 0.08 × 0.01 |
| 2θ range for data collection (°) | 6.58– 58.6 |
| h, k, l ranges | −15:13, −13:16, −18:12 |
| Reflections collected | 9733 |
| Independent reflections | 4386 |
| Rint | 0.0273 |
| Data / restraints / parameters | 4386 / 0 / 226 |
| Goodness-of-fit on F2 | 0.941 |
| Final R indices [I>2σ(I)] | R1 = 0.0605, wR2 = 0.1576 |
| R indices (all data) | R1 = 0.0772, wR2 = 0.1712 |
| Largest diff. peak and hole (e.Å−3) | 1.55 and −0.53 |
Table 2: Selected bond length (Å) and angles (°) for 1 with estimated standard deviations in parentheses.
| Distances | |||||
| Zn1–N1 | 2.182(4) | N7–O1 | 1.250(6) | ||
| Zn1–N3 | 2.179(3) | N7–O2 | 1.236(6) | ||
| Zn1–N4 | 2.152(5) | N7–O3 | 1.224(6) | ||
| Zn1–N5 | 2.206(4) | N8–O4 | 1.225(7) | ||
| Zn1–N6 | 2.217(4) | N8–O5 | 1.247(7) | ||
| N8–O6 | 1.235(7) | ||||
| Angles | |||||
| N1–Zn1–N2 | 82.76(19) | O1–N7–O2 | 121.2(4) | ||
| N1–Zn1–N4 | 90.76(16) | O1–N7–O3 | 120.6(4) | ||
| N1−Zn1−N5 | 103.35(16) | O2–N7–O3 | 118.1(4) | ||
| N1–Zn1–N6 | 88.57(15) | O4–N8–O5 | 120.9(5) | ||
| N5–Zn1–N6 | 78.27(18) | O4–N8–O6 | 118.3(6) | ||
| N5–Zn1–N4 | 87.4(2) | O5–N8–O6 | 120.7(5) | ||
Table 3. Dimensions of the hydrogen bonds (Å and °) in 1.
| D–H···A | d(D–H) | d(H···A) | <(DHA) | d(D···A) | Symmetry code |
| N1–H1A···O4 | 0.920 | 2.113 | 151.2 | 2.953(7) | x, y, z |
| N3–H3A···O2 | 0.921 | 2.102 | 174.6 | 3.020(6) | x, 0.5 − y, −0.5 + z |
| N3–H3B···O3 | 0.920 | 2.101 | 162.5 | 2.992(5) | x, y, z |
| N4–H4B···O6 | 0.920 | 2.121 | 167.1 | 3.025(7) | 1− x, 0.5 + y, 0.5 − z |
| N5–H5···O1 | 0.930 | 2.133 | 150.7 | 2.980(6) | x, 0.5 − y, −0.5 + z |
| N6–H6D···O1 | 0.920 | 2.126 | 178.3 | 3.046(6) | x, y, z |
Results and Discussion
The reactions between L and ethanolic solution of zinc(II) nitrate provided colorless crystals of 1. This complex was characterized by IR and Raman spectroscopy and X-ray crystallography. It is air-stable and soluble in DMSO.
Presence of the RNH2 group in 1 affect IR spectrum in two regions including 3313 and 3263 cm–1 for asymmetric and symmetric NH2 stretches and 1597 cm–1 for NH2 bending. Four bands in the IR spectrum near 1381, 1291, 1088, and 833 cm−1 for 1 can be assigned to vibrations of the nitrate groups (ν4, ν1, ν2 and ν6)9. The free nitrate ion has D3h symmetry and three infrared active vibrations, but this symmetry is lowered to C2v and Cs in metal complexes10, to give up to six infrared active vibrations. In 1, the nitrate groups are involved in hydrogen bonds lowering the symmetry. In the Raman spectrum of 1, a band at 600 cm–1 was assigned to the Zn–N stretching vibration.
Description of the crystal structure
The crystal structure of 1 was determined by X-ray single-crystal diffraction. The molecular graphics were drawn with ORTEP-III11 and Diamond 12.
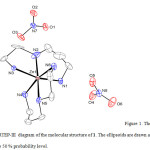 |
Figure 1: The ORTEP-III diagram of the molecular structure of 1. The ellipsoids are drawn at the 50 % probability level. Click here to View figure |
In the crystal structure of 1 (figure 1), zinc atom with coordination number six has a distorted octahedral geometry. Four sites are occupied by nitrogen atoms of the NH2 groups with the Cd–N bond lengths in the range of 2.152(5)–2.217(4) Å. The two other sites are occupied by nitrogen atoms of the NH groups with the Cd–N bond lengths in the range of 2.202(7)–2.206(4) Å. Two ligands in 1 which act as tridentate forming two five- and two six-membered chelate rings with the zinc atom and not any of them are planar. The dihedral angle between two N1/N2/N3/Zn1 and N4/N5/N6/Zn1 plans is 89.78° that confirm the mer form. If the distortion in the [ZnL2]2+ cation is disregarded, it has a C2 axis and C2 symmetry. The ligands have no chiral center, but two new one (N2, N5) are formed after coordination13 and have different enantiomeric forms. In the crystal network of 1 (figure 2), there are intermolecular N–H···O hydrogen bonds (table 3). The oxygen atoms in hydrogen bonds act as proton acceptors whereas the nitrogen atoms participate in hydrogen bonding as proton donors.
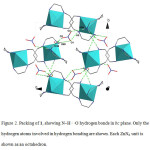 |
Figure 2: Packing of 1, showing N–H···O hydrogen bonds in bc plane. Only the hydrogen atoms involved in hydrogen bonding are shown. Each ZnN6 unit is shown as an octahedron. Click here to View figure |
Supplementary material
CCDC 917541 for mer–R,S-[ZnL2](NO3)2 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html.
Acknowledgement
We are grateful to Payame Noor University of I. R. Iran for financial support.
References
- Bartoszak-Adamska E., Bregier-Jarzebowska R. and Lomozik L., Polyhedron, 21, 739 (2002).
- Mondal A., Mostafa G., Ghosh A. and Chaudhuri N. R., J. Chem. Res., 570 (1998).
- Hakimi M., Maeder M. and Lawrance G. A., J. Coord. Chem., 64, 105 (2011).
- Hakimi M., Mardani Z., Moeini K., Minoura M. and Raissi H., Z. Naturforsch., 66b, 1122 (2011).
- Hakimi M., Mardani Z., Moeini K., Mohr F., Schuh E. and Vahedi H., Z. Naturforsch., 67b, 452 (2012).
- Hakimi M., Mardani Z., Moeini K. and Fernandes M. A., J. Coord. Chem., 65, 2221 (2012).
- Dolomanov O. V., Bourhis L. J., Gildea R. J., Howard J. A. K. and Puschmann H., J. Appl. Crystallogr., 42, 339 (2009).
- Sheldrick G. M., Acta Crystallogr., A46, 467 (1990).
- Hakimi M., Moeini K., Mardani Z., Mohr F. and Schuh E., J. Coord. Chem., accepted (2013).
- Hakimi M., Kukovec B.-M., Normohammadzadeh Z., Schuh E. and Mohr F., J. Chem. Crystallogr., 42, 180 (2012).
- Farrugia L. J., J. Appl. Crystallogr., 30, 565 (1997).
- Bergerhoff G., Berndt M. and Brandenburg K., J. Res. Natl. Inst. Stand. Technol., 101, 221 (1996).
- Hakimi M., Moeini K., Mardani Z., Fernandes M. A., Mohr F. and Schuh E., J. Coord. Chem. 65, 1232 (2012).

This work is licensed under a Creative Commons Attribution 4.0 International License.









