Justicia Adhatoda (Vasaka) Leaf Extracts as Eco-Friendly Corrosion Inhibitor for Mild Steel in Potable Water
K. S. Beenakumari
Department of Chemistry, All Saints’ College, Thiruvananthapuram, Kerala - 695 007, India. Corresponding Author E-mail: beenagireesh@yahoo.co.uk
The corrosion inhibition efficiency of the vasaka leaf extract for protecting mild steel in potable water (neutral aqueous medium) was studied by weight loss, Open circuit potential monitoring and potentiometric polarization methods. The inhibition test was performed in potable water rather than deionized water because inhibitors are normally applied in neutral and industrial waters. The extract of vasaka leaf has been found to show significant corrosion inhibition of mild steel in neutral aqueous environment. The inhibition efficiency increases with increasing the concentration of leaf extract and decreases with temperature. The inhibition efficiency calculated based on weight loss method was found to be above 95% when the electrolyte contain 700 ppm of the inhibitor. The results of different techniques used to measure the inhibition efficiency of the leaf extract are well co-related with each other.
KEYWORDS:Justicia adhatoda; Mild steel; Corrosion; Inhibitors
Download this article as:| Copy the following to cite this article: Beenakumari K. S. Justicia Adhatoda (Vasaka) Leaf Extracts as Eco-Friendly Corrosion Inhibitor for Mild Steel in Potable Water. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Beenakumari K. S. Justicia Adhatoda (Vasaka) Leaf Extracts as Eco-Friendly Corrosion Inhibitor for Mild Steel in Potable Water. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25224 |
Introduction
Mild steel pipes have been commonly used to supply potable water and corrosion of mild steel was one of the major problems in the supply network. The widely accepted technique to prevent the corrosion of the drinking water distribution systems is the use of suitable corrosion inhibitors. However, inhibitors to be used in potable water systems ought to be safe to human and friendly to the environment1. Currently research attention was given to natural products as corrosion inhibitors 2-5. The plant extracts have become important as an environmentally acceptable, readily available and renewable source for wide range of corrosion inhibitors 6,7. The present research work focused on developing a safe, environmental friendly and cheap corrosion inhibitor for protecting mild steel in drinking water systems. The inhibitor selected for the present study was the extract of the Justicia adhatoda (vasaka) leaf, which is environmental friendly, and also has some good medicinal value. The use of adhatoda leaf extract for corrosion prevention of mild steel in 0.5 M H2SO4 was already reported in literature8. The present work explores the possibility of using the vasaka leaf extract for protecting the mild steel in drinking water even at high temperatures.
Experimental
The specimens (3 x 2 x 0.1 cm) were produced from strips of mild steel (0.10 %C, 0.10 % Si, 0.70 %Mn, 0.02%Cr, 0.01%Ni, 0.10%Mo and balance Fe). The specimens were polished by using different metalo-graphic grade of emery papers, ultrasonically degreased by using isopropanol, cleaned by distilled water, dried in hot air and store in desiccators. The corrosion inhibitor used was the extract of Justicia adhatoda (vasaka) leaf. The extract of the leaf was collected by grinding and squeezing the leaf. The concentration of the inhibitor varies from 0 to 700 ppm (v/v). The temperature of the system varies from 30 oC to 80 oC. The turbulence was created by stirring the solution by using a magnetic stirrer, which rotates at a speed of 100 rpm. For each experiment fresh water solutions as well as freshly polished metallic samples were used. The experiment was duplicated to get the concordant results. Cathodic and anodic polarization curves were recorded on the metal electrode surface (1cm2) immersed in electrolyte medium by sweeping at a rate of 1 mV/s over a range of 100 mV vs. Saturated Calomel Electrodes (SCE). A Platinum foil (10 cm2) acts as counter electrode. The open circuit potential was measured as V vs SCE. The characteristics of water conducted for the study is given in table 1. The calcium and magnesium present in the water sample were analyzed by EDTA titration. The iron present in the water was analyzed by Atomic Absorption techniques using Atomic Absorption Spectrophotometer of Perkin Elmer (AA200). The anions like, chloride ion present in the medium was analyzed by titration with silver nitrate and sulphate by turbidimetric method using Thoshniwall turbidimeter. The metal constituents present on the scale formed on the surface of samples were analyzed by AAS. The carbon content present in the scale was estimated by using Leco- Carbon Analyzer.
Table 1: Characteristics of potable water.
| Sl. No. | Parameter | Quantity |
| 1 | pH | 6.5 |
| 2 | Residual chlorine (ppm) | 0.1 |
| 3 | Chloride (ppm) | 150 |
| 4 | Sulphate (ppm) | 100 |
| 5 | Iron (ppm) | 0.2 |
| 6 | Turbidity (NTU) | 5.0 |
| 7 | Total Hardness (as CaCO3 (ppm) | 250 |
| 8 | Calcium (ppm) | 70 |
| 9 | Magnesium (ppm) | 25 |
Results and Discussions
Open circuit potential decay
Figure 1 shows the open circuit potential (OCP) decay of mild steel specimen in potable water with different inhibitor concentration. The OCP value shifted to more anodic region with increase in the inhibitor concentration. The potential value shifted from –0.460 V to –0.100V by introducing 700 ppm of inhibitor. The anodic shift of the OCP value shows the high inhibition efficiency of the inhibitor. The OCP value of the mild steel with 700 ppm of inhibitor was found to be -0.230V at the time of introduction of the inhibitor, which shifted to anodic region with time and reaches to a value of -0.100 V at 24 hrs of immersion and remains steady for one months of immersion. At lower inhibitor concentration of 500 ppm, the anodic potential value after immersion of 24 hrs was found to be –0.160V, which remains steady for 10 days and decayed to cathodic regions. This shows that the inhibitor concentration of 500 ppm is not sufficient to protect the metal from corrosion on longer run. More over the inhibitor shift the potential to more anodic value indicates that it is an anodic inhibitor.
(Figure 1: OCP decay of mild steel samples in potable water contain different concentration of inhibitor at 30 oC, ● –0.0 ppm, ▲- 100 ppm, ○ – 300 ppm, ∆- 500 ppm, □ – 700 ppm)
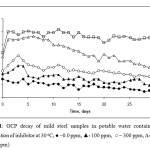 |
Figure 1: OCP decay of mild steel samples in potable water contain different concentration of inhibitor at 30 oC, ● –0.0 ppm, ▲- 100 ppm, ○ – 300 ppm, ∆- 500 ppm, □ – 700 ppm) |
The potential decay of mild steel with 700 ppm of inhibitor at higher temperature and stirring (turbulence) conditions are seen in figure 2. At high temperature of 80 oC, the OCP value was found to be –0.350V initially, which sifted to -0.172 V after duration of 24hrs and remains more or less steady up to one month of immersion. In stirred conditions and stirring at high temperature also shows same trend of OCP decay. With high temperature and turbulence, the OCP value required more delay to attain the steady value. Even after attaining the steady value, the OCP is found to be fluctuating with time. But all cases of immersion of mild steel samples with inhibitor, the OCP value was found to be more anodic when compared to blank under stagnant conditions (as in figure 1). This shows that the inhibitor is found to be very effective to shift the OCP value to more anodic region and prevent the corrosion of metal even at stimulated conditions.
(Figure 2: OCP decay of mild steel samples in potable water contain 700 ppm of inhibitor under different conditions ∆- stirring at 30 oC, ○ – heating at 80 oC, □ –stirring at 80 oC)
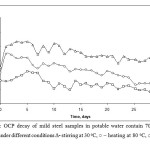 |
Figure 2: OCP decay of mild steel samples in potable water contain 700 ppm of inhibitor under different conditions ∆- stirring at 30 oC, ○ – heating at 80 oC, □ –stirring at 80 oC).
|
Polarization studies
The data obtained from potentiostatic polarization experiments were given in Table 2. The corrosion potential (Ecorr) shifted to more anodic value with increase in the concentration of the inhibitor. This shows the effectiveness of the inhibitor to shift the corrosion potential to more anodic passive region and thereby preventing the metal from corrosion. At higher temperature, the Ecorr value was found to be in cathodic regions compared to room temperature studies. This shows that corrosion rate is high at elevated temperature and at stirring conditions. But it was noticed that, even at high temperature and stirring conditions, the inhibitor loaded system is found to be more anodic compared to the blank system at stagnant conditions. The corrosion current density (icorr) decreases as the inhibitor concentration increases. The inhibitor efficiency is calculated using the equation IE (%) = (icorr at blank inhibitor – icorr at inhibitor ) X 100 icorr at blank inhibitor

Table 2: Polarization results of corrosion of mild steel in drinking water.
| Concentration of inhibitor (ppm) | Conditions | Ecorr (V vs SCE) | Icorr (μA/cm2) | IE (%) |
| 0 |
Stagnant at 30 oC |
-0.560 | 5.20 | |
| 100 | -0.520 | 3.50 | 32.7 | |
| 300 | -0.470 | 1.50 | 71.1 | |
| 500 | -0.380 | 1.00 | 80.7 | |
| 700 | -0.300 | 0.50 | 90.4 | |
| 700 | Stagnant at 80 oC | -0.370 | 0.80 | 84.6 |
| 700 | Stirred at 30 oC | -0.350 | 0.70 | 86.5 |
| 700 | Stirred at 80 oC | -0.390 | 1.00 | 80.7 |
The polarization results show that the vasaka leaf extract is found to be a very good corrosion inhibitor for protecting the mild steel in potable water even at stimulated conditions
Weight loss method
The ability of the inhibitor is determined by calculating the ratio of its efficiency to concentration in medium. Table 3 summarizes the weight loss experimental results conducted by immersing the mild steel coupens with and without inhibitor at different conditions. The weight loss experiments were conducted after 30 days of exposure of mild steel in water medium. The inhibition efficiency (IE) was calculated by using the formula
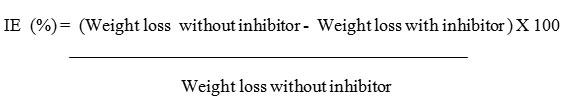
Table 3: Weight loss results of corrosion of mild steel in drinking water.
| Concentration of inhibitor (ppm) | Conditions | OCP (V vs SCE) | Corrosion rate (mpy) | IE (%) |
| 0 |
Stagnant at 30 oC |
-0.510 | 2.65 | 0 |
| 100 | -0.475 | 2.20 | 16.9 | |
| 300 | -0.412 | 1.12 | 53.9 | |
| 500 | -0.370 | 0.70 | 75.6 | |
| 700 | -0.235 | 0.12 | 95.5 | |
| 700 |
Stagnant at 80 oC |
-0.360 | 0.60 | 77.4 |
| 700 |
Stirred at 30 oc |
-0.290 | 0.50 | 81.1 |
| 700 |
Stirred at 80 oc |
-0.392 | 1.0 | 62.3 |
The OCP (at the time of immersion) values shifted to more anodic region with increase in inhibitor concentration. The anodic OCP value shows higher inhibition efficiency of the inhibitor. The corrosion rate decreases with increase in inhibitor concentration. The corrosion rate decreased to 0.12 mpy when the system contains 700 ppm of the inhibitor. The corrosion rate was found to be higher at stimulated conditions, but the values are very less compared to blank at stagnant conditions.
Mechanism of inhibition
The scale formed on the surface of the mild steel specimen immersed in electrolyte medium with inhibitor for 30 days of exposure was analyzed for its constituents and given in Table 4.
Table 4. Constituents present in the scale.
| Sl. No. | Constituents | Quantity (ppm) |
| 1 | Calcium | 720 |
| 2 | Magnesium | 400 |
| 3 | Organic substance as Carbon | 3000 |
| 4 | Iron oxide | balance |
The scratched sample contains calcium and magnesium as additional constituents with iron. The carbon content in the sample raveled the adsorption of inhibitor on metal surface. The calcium, magnesium and iron present in the water make complexes with the organic part present in the vasaka leaf. This complex will adhere on the surface of the mild steel and prevent the corrosion of the mild steel. In stimulated conditions, the complex that adheres on the surface will detached and the bare metal is exposed for the corrosive media and hence the corrosion rate was higher in stimulated conditions.
Conclusion
The vasaka leaf extract was found to be good eco-friendly inhibitor to prevent the corrosion of mild steel in drinking water environment. The inhibitor efficiency increased above 95% when the medium contains 700 ppm of the inhibitor. The inhibitor was found to be suitable for protecting the mild steel in stimulated conditions also. The inhibition efficiency calculated by weight loss method was found to be higher than that calculated on polarization studies. Polarization experiments were conducted just after 30 minutes of the immersion of mild steel sample in the electrolyte medium where as in weight loss method the observations were made after 30 days of exposure. A threshold time is needed for the inhibitor to start the inhibition, a slight variation in the inhibition efficiency was noted in the above two methods. The organic constituents present in vasaka leaf make complex with calcium, magnesium and iron present in water. This complex will adsorb on the metal surface and prevents the anodic oxidation of the metal.
Reference
- J. Buchweishaija, G. S. Mhinzi, Portugaliae Electrochimica Acta, 26: 257 (2008).
- L. R. Chauhan, G. Gunasekaran, Corrosion Science, 49: 1143 (2007).
- M. V. Sheyreese, B. O. Cyril, J. Corrosion Sci. Eng. ,7: 36 ( 2005).
- G. N. Mehta, A. V. Smita, Bull. Electrochem., 15: 67 (1999).
- K. S. Beenakumari, Green Chem. Lett. and Rev.,4: 117 (2011).
- P. B. Raja, M. Sethuraman, Material Letters, 62: 113 (2008).
- K. S. Beenakumari, Anal. & Bioanal.Electrochem., 2: 36 (2010).
- M. Ramananda Singh, J. Mater. Environ. Sci.,4: 119 (2013 issue ).

This work is licensed under a Creative Commons Attribution 4.0 International License.









